1. Давыдов М.И., Полоцкий Б.Е. Рак легкого. М.: Радикс, 1994.
2. Трахтенберг А.Х., Чиссов В.И. Клиническая онкопульмонология. М.: ГЭОТАР-Медиа, 2000.
3. The health consequences of smoking: a report of the Surgeon General. Доступно по: https://www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smokingfactsheet/index.html.
4. Под ред. А.Д. Каприна, В.В. Старинского, А.О. Шахзадовой. Состояние онкологической помощи населению России в 2022 году. - М.: МНИОИ им. П.А. Герцена - филиал ФГБУ "НМИЦ радиологии" Минздрава России, 2022. - илл. - 239 с. Доступно по: https://oncology-association.ru/wp-content/uploads/2023/08/sop-2022-el.versiya_compressed.pdf
5. Stahel R., Peters S., Garassino M. Thoracic tumours essentials for clinicians. ESMO Press, 2014.
6. WHO Classification of Tumours Editorial Board. Thoracic tumours. Lyon (France): International Agency for Research on Cancer; 2021. (WHO classification of tumours series, 5th ed.; vol. 5).
7. The AJCC Cancer staging system. 9thmanual. Eight ed. Springer, 20242017.
8. Методическое пособие "Рак легкого", Кононец П.В., 2014, с. 45
9. Рак легкого/Под ред. К. К. Лактионова и В.В. Бредера. 2-е изд., испр. и доп. - М.: ГРАНАТ, 2021. - 192 с.
10. Grunnet M., Sorensen J.B., Carcinoembrionic antigen (CEA) as a tumor marker in lung cancer. Lung Cancer 2012; 76: 138-43.
11. Planchard D., Popat S., Kerr R. et al. Metastatic non-small-cell lung cancer: ESMO clinical practiceguidelines for diagnosis, treatment and follow-up. Ann of Oncology 2018; 29 (Suppl. 4): 192 - 234.
12. NCCN Guidelines. Non-small cell lung cancer, version 3.2023. Available at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
13. Travis D.W., Brambilla E., Burke A.P. et al. Classification of tumours of the lung, pleura, thymusand heart. Lyon: IARC Press, 2015.
14. Lung cancer. Ed. by F.V. Fossella, J.B. Putnam, R. Komaki. NewYork: Springer, 2003.
15. Schrump D.S., Carter D., Kelsey C.R. et al. Non-small cell lung cancer. In: DeVita, Hellman, and Rosenberg cancer; prinsiples and practice of oncology. Ed. by V.T. DeVita Jr, T.S. Lawrence, S.A. Rosenberg et al. Philadelphia: Lippincott Williams and Wilkins, 2011.
16. Трахтенберг А.Х., Чиссов В.И., Франк Г.А. Нейроэндокринные опухоли легких. М.: Практическая медицина, 2012.
17. Аллахвердиев А.К., Давыдов М.М. Торакоскопическая лобэктомия с медиастинальной лимфодиссекцией стандарт в хирургическом лечении больных немелкоклеточным раком легкого Т1-2N0M0. Вопросы онкологии 2015; 61(3): 413-7.
18. Higuchi M., Yaginuma H., Yonechi A. et al. Long-term outcomes after video-assisted thoracicsurgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage Ia non-small cell lung cancer. J Cardiothorac Surg 2014; 9: 88 - 92.
19. Lindberg K., Nyman J., Riesenfeld Kallskog V. et al. Long-term of a results of prospective trialphase II of medically inoperable stage I NSCLC treated with SBRT - the Nordic experience. Acta Oncol 2015; 54: 1096-104.
20. Zhang T., Guo Q., Zhang Y. et al. Meta-analysis of adjuvant chemotherapy versus surgery alone inT2aN0 stage IB non-small cell lung cancer. J Can Res Ther 2018; 14: 139-44.
21. Winton T., Livingston R., Johnson D. et al. Vinorelbine plus cisplatinvs observation in resectednon-small-cell lung cancer. N Engl J Med 2005; 352: 2589-97.
22. Arriagada R., Dunant A., Pignon J.P. Long-term results of the international adjuvant lung cancertrial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010; 28(1): 35 - 42. doi: 10.1200/JCO.2009.23.2272
23. NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010; 375(9722): 1267-77.
24. Aureperin A., Le Pechoux C., Rolland E. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small cell lung cancer. J Clin Oncol 2010; 28: 2181-90.
25. Tsitsias T., Boulemden A., Ang K. et al. The N 2 paradox: similar outcomes of pre- and postoperatively identified single-zone N 2a positive non-small-cell lung cancer. J Eur Cardiothorac Surg 2014; 45: 882-7.
26. Scagliotti G.V., Pastorino U., Vansteenkiste J.F. et al. Randomized phase III study of surgery aloneor surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012; 30: 172-8.
27. Usami N., Yokoi K., Hasegawa Y. et al. Phase II study of carboplatin and gemcitabine as adjuvantchemotherapy in patients with completely resected non-small cell lung cancer: a report from the Central Japan Lung Study Group, CJLSG 0503 trial. Int J Clin Oncol 2010; 15: 583-7.
28. 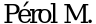 , Chouaid C.,
, Chouaid C., 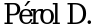 et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30: 3516-24.
et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30: 3516-24.
29. Gilligan D., Nicolson M., Smith I. et al. Preoperative chemotherapy in patients with resectablenon-small cell lung cancer: results of the MRC LU22/NVALT2/EORTC 08012 multicentre randomised trial and update of systematic review. The Lancet 2007; 369(9577): 1929-37. doi: 10.1016/S01406736(07) 60714-4.
30. Strauss G.M., Herndon III J.E., Maddaus M.A. et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008; 26: 5043-51.
31. Fossella F., Pereira J.R., von Pawel J. et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003; 21: 3016-24.
32. Burdett S., Rydzewska L., Tierney J. et al. Postoperative radiotherapy for non-small cell lungcancer. Cochrane Database Syst Rev 2016; 10(10): CD002142. doi: 10.1002/14651858.CD002142.pub4.
33. Arriagada R., Bergman B., Dunant A. et al. International Adjuvant Lung Cancer Trial CollaborativeGroup. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004; 350(4): 351-60.
34. Kreuter M., Vansteenkiste J., Fishcer J.R. et al. Randomized phase 2 trial on refinement of earlystage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Ann Oncol 2013; 24: 986-92.
35. Curran W.J., Paulus R., Langer C.J. et al. Sequential vs concurrent chemoradiation for stage IIInon-small-cell lung cancer randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452-60.
36. Pignon J.P., Tribodet H., Scagliotti G.V. et al. Lung adjuvant cisplatin evaluation: a pooled analysisby the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552-9.
37. Gridelli С., Chen T., Ko A. et al. nab-Paclitaxel/carboplatin in elderly patients with advanced squamous non-small cell lung cancer: a retrospective analysis of a Phase III trial. Drug Des Devel Ther 2018; 12: 1445-51. doi: 10.2147/DDDT.S155750.
38. Choy H., Gerber D.E., Bradley J.D. et al. Concurrent pemetrexed and radiation therapy in thetreatment of patients with inoperable stage III non-small cell lung cancer: a systematic review of completed and ongoing studies. Lung Cancer 2015; 87: 232-40.
39. Senan S., Brade A., Wang L.H. et al. PROCLAIM: randomized phase III trial of pemetrexedcisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2016; 34: 953-62.
40. Albain K.S., Crowley J.J., Turrisi A.T. III et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group Phase II Study, SWOG 9019. J Clin Oncol 2002; 20: 3454-60.
41. Bradley J.D., Paulus R., Komaki R. et al. Standard-dose versus high-dose conformal radiotherapywith concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187-99.
42. Antonia S.J., Villegas A., Daniel D. et al. Durvalumab after chemoradiotherapy in stage III nonsmall-cell lung cancer. N Engl J Med 2017; 377(20): 1919-29.
43. Masters G.A., Temin S., Azzoli C.G. et al. Systemic therapy for stage IV non-small cell lung cancer. American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2015; 33: 3488-515.
44. Kerr K.M., Bubendorf L., Edelman M.J. et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 2014; 25: 1681-90.
45. Rosell R., Carcereny E., Gervais R. et al. Erlotinib versus standard chemotherapy as first-linetreatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239-46.
46. Yang J.C., Wu Y.I., Shuler M. et al. Afatinib versus cisplatin-based chemotherapy for EGFRmutation- positive lung adenocarcinoma LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomized, phase 3 trials. Lancet Oncol 2015; 16: 141-51.
47. Soria J.C., Ohe Y., Vansteenkiste J. et al. Osimertinib in untreated EGFR mutated advanced nonsmall-cell lung cancer. N Engl J Med 2018;378:113-25.
48. Solomon B.J., Mok T., Kim D.W. et al. First-line crisotinib versus chemotherapy in ALK-positivelung cancer. N Engl J Med 2013;371:2167-77.
49. Soria J.C., Tan D.S.W., Chiari R. et al. First-line ceritinib versus platinum-based chemotherapy inadvanced ALK-rearranged non-small-cell lung cancer (ASCEND 4): a randomized, open-label, phase 3 study. Lancet 2017; 389: 917-29.
50. Camidge D.R. Updated efficacy and safety data from the global phase III ALEX global study ofalectinib (ALC) versus crizotinib (CZ) in untreated advanced ALK+ NSCLC. J Clin Oncol 2018; 35(Suppl): 9064.89
51. Planchard D., Smit E.F., Groen H.J.M. et al. Dabrafenib plus trametinib in patients with previouslyuntreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017; 18: 1307-16.
52. Zhao X., Suryawanshi S., Hruska M. et al. Assessment of nivolumab benefit-risk profile of a 240mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Annals of Oncology 2017; 28(8): 2002-8.
53. Klastersky J., Sculier J.P., Lacroix H. et al. A randomized study comparing cisplatin or carboplatinwith etoposide in patients with advanced non-small cell lung cancer: European Organization for Research and Treatment of Cancer Protocol 07861. J Clin Oncol 1990; 8: 1556-62.
54. Frasci G., Comella P., Panza N. et al. Carboplatin-oral etoposide personalized dosing in elderlynon-small cell lung cancer patients. Gruppo Oncologico Cooperativo Sud-Italia. Eur J Cancer 1998; 34: 1710-4.
55. Ohe Y., Ohashi Y., Kubota K. et al. Randomized phase III study of cisplatin plus irinotecan versuscarboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 2007; 18: 317-23.
56. Sandler A., Gray R., Perry M.C. et al. Paclitaxel-carboplatin alone or with bevacizumab for nonsmall cell lung cancer. N Engl J Med 2006; 355: 2542-50.
57. Scagliotti G.V., Parikh P., von Pawel J. et al. Phase III study comparing cisplatin plus gemcitabinewith cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage NSCLC. J Clin Oncol 2008; 26: 3543-51.
58. Danson S., Middleton M.R., O'Byrne K.J. et al. Phase III trial of gemcitabine and carboplatinversus mitomycin, ifosfamide, and cisplatin or mitomycin, vinblastine, and ciplatin in patients with advanced non-small cell lung carcinoma. Cancer 2003; 98: 542-53.
59. Barlesi F., Scherpereel A., Rittmeywr A. et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small cell lung cancer: AVAPERL. J Clin Oncol 2013; 31: 3004-11.
60. Takagi Y., Hosomi Y., Sunami K. et al. A Prospective Study of Shortened Vitamin Supplementation Prior to Cisplatin-Pemetrexed Therapy for Non-Small Cell Lung Cancer. Oncologist 2014; 19(11): 1194-9. doi: 10.1634/theoncologist.2014-0221.
61. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individualpatients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995; 311: 899 - 909.
62. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010; (5): CD007309.
63. Azzolic G., Temin S., Aliff T. et al. 2011 Focused Update of 2009 American Society of ClinicalOncology clinical practice guideline update of chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2011; 29: 3825-31.
64. D'Addario G., Pintile M., Leighi N.B. et al. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of published literature. J Clin Oncol 2005; 23: 2926-36.
65. Zukin M., Barrios C.H., Pereira J.R. et al. Randomized phase III trial of single-agent pemetrexedversus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013; 31: 2849-53.
66. Переводчикова Н.И., В.А. Горбунова Руководство по химиотерапии опухолевых заболеваний. М.: Практическая медицина, 2015.
67. Gridelli C., Perrone F., Gallo C. et al. Chemotherapy in elderly patients with advanced non-smallcell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 2003; 95: 362-72.
68. Corre R., Greillier L., Le Caer H. et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced nonsmall-cell lung cancer: the phase III randomized ESOGIA-GFPC-GECP 08-02 study. J Clin Oncol 2016; 34: 1476-83.
69. Henry H.D., Costa I., Goldwasser F. et al. Randomised, double-blind study of denosumab versus zoledronic acid in the treatment skeletal metastases in patients with advanced cancer (excluded breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125-32.
70. Zatloukal P., Kanitz E., Magyar P. et al Gemcitabine in locally advanced and metastatic non- smallcell lung cancer: the Central European phase II study. Lung Cancer 1998; 22: 243-50.
71. Perol M., Chouaid C., Perol D. et al. Randomized, phase III study of gemcitabine or erlotinibmaintenance therapy versus observation, with predefined second-line treatment, after cisplatingemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30: 3516-24.
72. Fossella F.V., DeVore R., Kerr R.N. et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18: 2354-62.
73. Fidias P.M., Dakhil S.R., Lyss A.P. et al. Phase III study of immmediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small cell lung cancer. J Clin Oncol 2009; 27: 591-8.
74. Hanna N.H., Sheperd F.A., Fossella F.V. et al. Randomized phase III study of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589-97.
75. Rosen L.S., Gordon D., Tchekmedyian N.S. et al. Long-term efficacy and safety of zoledronic acidin the treatment of skeletal metastases in patients with non-small-cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer 2004; 100: 2613-21.
76. Cardenal F., 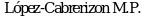 ,
, 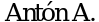 et al. Randomized phase III study of gemcitabine cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 1999; 17(1): 12-8.
et al. Randomized phase III study of gemcitabine cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 1999; 17(1): 12-8.
77. Chow E., Zeng L., Salvo N. et al. Update on the systematic review of palliative radiotherapy trialsfor bone metastases. Clin Oncol (R Coll Radiol) 2012; 24: 112-24.
78. Reck M., Rodriguez-Abreu D., Robinson A.G. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823-33. doi: 10.1056/NEJMoa1606774.
79. Lopes G., Wu Y.-L., Kudaba I. et al. Pembrolizumab versus platinum-based chemotherapy as firstline therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score ![]() 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 2018; 36(18 Suppl).
1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 2018; 36(18 Suppl).
80. Langer C.J., Gadgeel S.M., Borghaei H. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17(11): 1497-508.
81. Paz-Ares L., Luft A., Vicente D. et al. Pembrolizumab plus chemotherapy for squamousnon-small-cell lung cancer. N Engl J Med 2018; 379(21): 2040-51.
82. Socinski M.A., Jotte R.M., Cappuzzo F. et al. Atezolizumab in first-line treatment of metastaticnonsquamous NSCLC. N Engl J Med 2018; 378: 2288-301.
83. Gandhi I., Rodriguez-Abreu D., Gadgeel S. et al. Pembrolizumab plus chemotherapy in metastaticnon-small-cell lung cancer. N Engl J Med 2018; 378: 2078-92.
84. Sheperd F.A., Dancey J., Ramlau R. et al. Prospective randomized trial of docetaxel versus bestsupportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18: 2095-103.
85. Brahmer J., Reckamp K.L., Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cellnon-small-cell lung cancer. N Engl J Med 2015; 373: 123-35.
86. Borghaei H., Paz-Ares L., Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamousnon-small-cell lung cancer. N Engl J Med 2015; 373: 1627-39.
87. Rittmeyer A., Barlesi F., Waterkamp D. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255-65.
88. Herbst R.S., Baas P., Kim D.W. et al. Pembrolizumab versus docetaxel for previously treated, PDL1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540-50.
89. Herbst R., Garon E., Kim D.-W. et al. OA03.07 KEYNOTE-010: durable clinical benefit in patients with previously treated, PD-L1-expressing NSCLC who completed pembrolizumab. J Thoracic Oncol 2017; 12: 254-5.
90. Long G.V., Tykodi S.S., Schneider J.G. et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Annals of Oncology 2018; 29(11): 2208-13.
91. Garon E., Reck M., Rodriguez-Abreu D. et al. P3.02c-030 Use of a 200-Mg Fixed Dose of Pembrolizumab for the Treatment of Advanced Non-Small Cell Lung Cancer (NSCLC): Topic: IT/JTO 2017; 12(1): S:S1290-91 doi: https://doi.org/10.1016/j.jthe.2016.11.1825.
92. Morrissey K.M., Marchand M., Patel H. et al. Alternative dosing regimens for atezolizumab: anexample of model-informed drug development in the postmarketing setting. Cancer Chemother Pharmacol 2019; 84(6): 1257-67. doi: 10.1007/s00280-019-03954-8. Epub 2019 Sep 21.
93. Garassino M., Martelli O., Broggini M., Farina G. et al. Erlotinib versus docetaxel as second-linetreatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet oncol 2013; 14(10): 981-8.
94. Novello S., Kaiser R., Mellengaard A. et al. Analysis of patient-reported outcomes from the LUME-Lung I trial: a randomized, double-blind, placebo-controlled. Phase III study of second line nintedanib in patients non-small-cell lung cancer. Eur J Cancer 2015; 51: 317-26.
95. Sculier J.P., Lafitte J.J., Berghmans T. et. al. A phase II trial testing gemcitabine as second-linechemotherapy for non-small-cell lung cancer. The European Lung Cancer Working Party. Lung Cancer 2000; 29: 67 - 73.
96. Ciuleanu T., Stelmakh L., Cicenas S. et al. Efficacy and safety of erlotinib versus chemotherapy insecond-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012; 13: 300-8.
97. Karampeazis A., Voutsina A., Souglakos J. et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer 2013; 119: 2754-64.
98. Soria J.C., Felip E., Cobo M. et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16: 897 - 907.
99. Planchard D., Popat S., Kerr K. Metastatic non-small cell lung cancer: ESMO Clinical PracticeGuidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29 (Suppl 4): iv192-237.
100. Ahn M.J., Kim S.W., Cho B.C. et al. Phase II study of Afatinib as third-line treatment for patientsin Korea with stage IIIB/IV non-small cell lung cancer harboring wild-type EGFR. Oncologist 2014; 19(7): 702-3.
101. Cho B.C., Kim D.W., Bearz A. et al. ASCEND-8 - a randomized phase I study ceritinib 450 mg or600 mg taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK) rearranged metastatic non-small-cell lung cancer (NSCLC). J Thorac Oncol 2017; 12(9): 1357-67. doi: 10.1016/j.jtho.2017.07.005.
102. Inoue A., Kobayashi K., Usui K. et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J ClinOncol 2009; 27: 1394-400.
103. Riely G.J., Yu H.A. EGFR: The Paradigm of an Oncogene-Driven Lung Cancer. Clin Cancer Res2015; 21(10): 2221-6. doi: 10.1158/1078-0432.CCR-14-3154.
104. Eisenhauer E.A., Therasse P., Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228-47.
105. Miller A.B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer 1981; 47(1): 207-14.
106. Burroto M., Manasanch E.E., Wilkerson J., Fojo T. Gefitinib and erlotinib in metastatic nonsmall-cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015; 20: 400-10.
107. Sequst L.V., Yang J.C., Yamamoto N. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327-34.
108. Cross D.A., Ashton S.E., Ghiorghiu S. et al. AZD9291 irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014; 4: 1046-61.
109. Oken M.M., Creech R.H., Tormey D.C. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982, 5(6): 649-55.
110. Oxnard G.R., Thress K.S., Alden R.S. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016; 34(28): 3375-82. doi: 10.1200/JCO.2016.66.7162.
111. Shaw A.T., Ou S.H., Bang Y.J. et al. Crizotinib in ROS1-rearranged nonsmall-celllung cancer. N Engl J Med 2014; 371: 1963-71.
112. Shaw A.T., Yeap B.Y., Solomon B.J. et al. Impact of crisotinib on survival in patients with advanced, ALK-positive NSCLC compared with historical controls [abstract]. J Clin. Oncol 2011; 29(Suppl. 15):7507.
113. Moro-Sibilot D., Faivre L., Zalcman G. et al. Crizotinib in patients with advanced ROS1rearranged non-small cell lung cancer (NSCLC). Preliminary results of the ACSE phase II trial. J Clin Oncol 2015; 33: abstr 8065.
114. Mazieres J., Zalcman G., Crino L. et al. Crizotinib therapy for advanced lung adenocarcinomaand a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015; 33: 992-9.
115. Pan J. Dabrafenib Plus Trametinib for BRAF V600E-Mutant Non-small Cell Lung Cancer: APatient Case
Report. Clinical Drug Investigation 2019; 39: 1003-7.
116. Planchard D., Smit E.F., Groen H.J.M. et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer:an open-label, phase 2 trial. Lancet Oncol 2017; 18: 1307-16.
117. Planchard D., Besse B., Groen H.J.M. et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic nonsmall cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016; 17(7): 984-93.
118. Planchard D., Smit E.F., Groen H.J.M. et al. Updated survival of patients (pts) with previouslytreated BRAF V600E-mutant advanced non-small cell lung cancer (NSCLC) who received dabrafenib (D) or D+ trametinib (T) in the phase II BRF113928 study. J Clin Oncol 2017; 35 (Suppl.); abstract 9075.
119. Butts C.A., Ding K., Seymour L. et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010; 28: 29 - 34.
120. Sandler A., Yi J., Dahlberg S. et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacisumab with paclitaxel/carboplatin for advanced non-smallcell lung cancer. J Thorac Oncol 2010; 5: 1416-23.
121. Yang B., Lee H., Um S.W. et al. Incidence of brain metastasis in lung adenocarcinoma at initialdiagnosis on the basis of stage and genetic alterations. Lung Cancer 2019; 129: 28 - 34.
122. Patchell R.A., Tibbs P.A., Walsh J.W. et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med 1990; 322:494-500.
123. Noordijk E.M., Vecht C.J., Haaxma-Reiche H. et al. The choice of treatment of single brainmetastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994; 29: 711-7.
124. Sahgal A., Aoyama H., Kocher M. et al. Phase 3 trials of stereotactic radiosurgery with or withoutwhole-brain radiation therapy for 1 to 4 brain metastases: individual patient data of meta-analysis. Int J Radiat Oncol Biol Phys 2015; 91: 710-7.
125. Priestman T.J., Dunn J., Brada M. et al. Final results of the Royal College of Radiologists' Trialcomparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol 1996; 8: 308-15. 124. Batchelor T., DeAngelis L.M. Medical management of cerebral metastases. Neurosurg Clin NorthAm 1996; 7: 435-46.
126. Naruke T. Lymph node metastasis of lung cancer and associated surgery. Asian Med J 1990; 33(12): 668-77.
127. Paul S., Altorki N.K., Sheng S. et al. Thoracoscopic lobectomy is associated with lower morbiditythan open lobectomy: a propensity-matched analysis from the STS Database. J Thorac Cardiovasc Surg 2010; 139:366-78.
128. Scott W.J., Allen M.S., Darling G. et al. Video-assisted thoracic surgery versus open lobectomyfor lung cancer: a secondary analysis of data from the American College of Sugeons Oncology Group Z0030 randomised clinical trial. J Thorac Cardiovasc Surg 2010; 139: 976-81.
129. Ginsberg R.J., Rubinstein L.V. Randomised trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg 1995; 60: 615-22.
130. Schreiber D., Rineer J., Weedon J. et al. Survival outcomes with the use of surgery in limitedstage small cell lung cancer: should its role be re-evaluated? Cancer 2010; 116(5): 1350-7.
131. Мелкоклеточный рак легкого. Под ред. М.Б. Бычкова. М.: Фармарус принт Медиа, 2013.
132. De Ruysscher D., Pijls-Johannesma M., Vansteenkiste J. et al. Systematic review and metaanalysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limitedstage, small-cell lung cancer. Ann Oncol 2006; 17: 543-52.
133. Turrisi A.T., Kim K., Blum R. et al. Twice-daily compared with once-daily thoracic radiotherapyin limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 340: 265-71. 133. Zatloukal P., Cardenal F., Szczesna A. et al A multicenter international randomized phase III studycomparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease Ann Oncol 2010; 21(9): 1810-6.
134. Rossi A., Di Maio M., Chiodini P. et al. Carboplatin- or cisplatin-based chemotherapy in first-linetreatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012; 30(14): 1692-8.
135. Horn L., Mansfeld A.S., Szcz sna A. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220-9.
136. Von Pawel J., Schiller J.H., Shepherd F.A., Fields S.Z. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999; 17(2): 658-67.
137. Morise M., Niho S., Umemura S. et al. Low-dose irinotecan as a second-line chemotherapy forrecurrent small cell lung cancer. Jpn J Clin Oncol. 2014; 44(9): 846-51. doi:10.1093/jjco/hyu094. Epub 2014 Jul 23.
138. Yamamoto N., Tsurutani J., Yoshimura N. et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res. 2006; 26(1B): 777-81.
139. Smit E., Fokkema E., Biesma B. et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998; 77(2): 347-51.
140. Slotman B., Faivre-Finn C., Kramer G. et al. EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007; 357(7): 664-72.
141. Slotman B.J., van Tinteren H., Praag J.O. et al. Use of thoracic radiotherapy for extensive stagesmall cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015; 385: 36-42.
142. Sommer M.S., Trier K., Vibe-Petersen J. et al. Perioperative rehabilitation in operation for lungcancer (PROLUCA) - rationale and design. BMC Cancer 2014; 14: 404. doi: 10.1186/1471-2407-14404.
143. Sebio R.,  ,
,  et al. Impact of a pre-operative pulmonaryrehabilitation program on functional performance in patients undergoing video-assisted thoracic surgery for lung cancer. Arch Bronconeumol 2016; 52(5): 231-2.
et al. Impact of a pre-operative pulmonaryrehabilitation program on functional performance in patients undergoing video-assisted thoracic surgery for lung cancer. Arch Bronconeumol 2016; 52(5): 231-2.
144. Sebio G.R., Y ez Brage M.I., Gim nez Moolhuyzen E. et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2016; 23(3): 486-97.
145. Barassi G., Bellomo R.G., Di Iulio A. et al. Preoperative rehabilitation in lung cancer patients: yoga approach. Adv Exp Med Biol 2018; 1096: 19 - 29.
146. Rogers L.J., Bleetman D., Messenger D.E., Joshi N.A. et al. The impact of enhanced recoveryafter surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018; 155(4): 1843-52.
147. Li S., Zhou K., Che G. et al. Enhanced recovery programs in lung cancer surgery: systematicreview and meta-analysis of randomized controlled trials. Cancer Manag Res 2017 Nov 16; 9: 657-70. doi: 10.2147/CMAR.S150500.
148. Deng G.E., Rausch S.M., Jones L.W. et. al. Complementary therapies and integrative medicine inlung cancer. diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013; 143(5 Suppl): e420S-36S.
149. Imperatori A., Grande A., Castiglioni M. et al. Chest pain control with kinesiology taping afterlobectomy for lung cancer: initial results of a randomized placebo-controlled study. Interact Cardiovasc Thorac Surg 2016; 23: 223-30.
150. Park H., Park J., Woo S.Y. et al. Effect of high-frequency chest wall oscillation on pulmonaryfunction after pulmonary lobectomy for non-small cell lung cancer. Crit Care Med 2012; 40(9): 2583-9.
151. Dhillon H.M., van der Ploeg H.P., Bell M.L. et al. The impact of physical activity on fatigue andquality of life in lung cancer patients: a randomised controlled trial protocol. BMC Cancer 2012; 12: 572.
152. Schmitz K.H., Courneya K.S., Matthews C. et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010; 42: 1409-26.
153. Janssen S.M., Abbink J.J., Lindeboom R., Vliet Vlieland T.P. Outcomes of pulmonary rehabilitation after treatment for non-small cell lung cancer stages I to IIIa: an observational study. J Cardiopulm Rehabil Prev 2017; 37(1): 65-71.
154. Sun V., Raz D.J., Ruel N. et al. A multimedia self-management intervention to prepare cancerpatients and family caregivers for lung surgery and postoperative recovery. Clin Lung Cancer 2017; 18(3): e151-9.
155. Batty G.D., Russ T.C., Stamatakis E., Kivim ki M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ 2017; 356: j108.
156. Luszczynska A., Paw owska I., Cieslak R. et al. Social support and quality of life among lungcancer patients: a systematic review. Psychooncology 2013; 22(10):2160-8.
157. Li M., Kennedy E., Byrne N. et al. Systematic review and meta-analysis of collaborative careinterventions for depression in patients with cancer. Psycho-Oncology. 2017; 26: 573-87.
158. Rivas-Perez H., Nana-Sinkam P. et al. Integrating pulmonary rehabilitation into the multidisciplinary management of lung cancer: a review. Respir Med 2015; 109(4): 437-42.
159. Andrea L., Kollasch J., Vandenberg J. et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage 2013; 45: 811-21.
160. Hilliard R.E. Music therapy in hospice and palliative care: a review of the empirical data. EvidBased Complement Alternat Med 2005; 2(2): 173-8.
161. Chen H.Y., Li S.G., Cho W.C.S., Zhang Z.J. The role of acupoint stimulation as an adjunct therapy for lung cancer: a systematic review and meta-analysis. BMC Complement Altern Med 2013; 13: 362.
162. Streckmann F., Zopf E.M., Lehmann H.C. et al: Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med 2014; 44: 1289-304.
163. Muzi J.L., Look R.M., Turner C. et al. Low-level laser therapy for chemotherapy-induced peripheral neuropathy. J Clin Oncol 2012; 30(15 Suppl): 9019.
164. Rick O., von Hehn U., Mikus E. et al. Magnetic field therapy in patients with cytostatics-inducedpolyneuropathy: A prospective randomized placebo-controlled phase-III study. Bioelectromagnetics 2016; 38(2): 85-94.
165. Kilin M., Livanelioglu A., Yildirim S.A., Tan E. Effects of transcutaneous electrical nerve stimulation in patients with peripheral and central neuropathic pain. J Rehabil Med 2014; 46(5): 454-60.
166. Oberoi S., Zamperlini-Netto G., Beyene J. et al. Effect of prophylactic low level laser therapy onoral mucositis: a systematic review and meta-analysis. PLoS One 2014; 9(9): e107418.
167. Ross M., Fischer-Cartlidge E. Scalp cooling: a literature review of efficacy, safety, and tolerability for chemotherapy-induced alopecia. Clin J Oncol Nurs 2017; 21(2): 226-33.
168. Hetkamp M., Bender J., Rheindorf N. et al. A Systematic Review of the Effect of Neurofeedbackin Cancer Patients. Integr Cancer Ther 2019; 18: 1534735419832361. doi:10.1177/1534735419832361.
169. Bade B.C., Thomas D.D., Scott J.B., Silvestri G.A. Increasing physical activity and exercise inlung cancer. reviewing safety, benefits, and application. J Thorac Oncol 2015; 10(6): 861-71.
170. Bensadoun R.J., Nair R.G. Low-level laser therapy in the management of mucositis and dermatitis induced by cancer therapy. Photomed Laser Surg 2015; 33(10): 487-91.
171. Williams S., Dale J. The effectiveness of treatment for depression/depressive symptoms in adultswith cancer: a systematic review. Br J Cancer 2006; 94: 372-90.
172. Karnofsky D.A., Burchenal J.H. The clinical evaluation of chemotherapeutic agents in cancer. In: Evaluation of chemotherapeutic agents. Edn. Ed. by MacLeod C. New York: Columbia University Press, 1949.191-205 с.
173. Hendriks L.E.L. et al. Effect of bisphosphonates, denosumab, and radioisotopes on bone pain andquality of life in patients with non-small cell lung cancer and bone metastases: A systematic review. J Thorac Oncol 2016; 11(2): 155-73.
174. Ettinger D.S. et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw Harborside Press, LLC, 2017; 15(4): 504-35.
175. Rolfo C., Mack P.C., Scagliotti G.V. et al. Liquid Biopsy for Advanced Non-Small Cell LungCancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol 2018; 13(9): 1248-68.
176. Merker J.D., Oxnard G.R., Compton C. et al. Circulating Tumor DNA Analysis in Patients WithCancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 2018; 36(16): 1631-41.
177. Oxnard G.R., Thress K.S., Alden R.S. et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016; 34(28): 3375-82.
178. Sacher A.G., Paweletz C., Dahlberg S.E. et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2016; 2(8): 1014-22.179.
179. Kate S., Chougule A., Joshi A. et al. Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: a single center retrospective analysis. Lung Cancer (Auckl) 2019; 10: 1-10.
180. Cho J.H., Lim S.H., An H.J. et al. Osimertinib for Patients With Non-Small-Cell Lung CancerHarboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020; 38(5): 488-95.
181. Zhang T., Wan B., Zhao Y. et al. Treatment of uncommon EGFR mutations in non-small cell lungcancer: new evidence and treatment. Transl Lung Cancer Res 2019; 8(3): 302-16.
182. Passaro A., Pochesci A., Spitaleri G. et al. Afatinib in first-line setting for NSCLC harbouringcommon EGFR mutations: new light after the preliminary results of LUX-Lung 7. J Thorac Dis 2016; 8(3): E217 - E220.
183. Takeda Y., Naka G., Yamaguchi Y. et al. Genetic diagnostic features after failure of initial treatment with epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors among non-smallcell lung cancer patients harboring EGFR mutations. BMC Cancer 2020; 20(1): 951. doi: 10.1186/s12885-020-07424-w.
184. NCCN Guidelines. Non-small cell lung cancer, version 6.2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf.
185. Chan T.A., Yarchoan M., Jaffee E. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019; 30(1): 44 - 56.
186. Luchini C., Bibeau F., Ligtenberg M.J.L. et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019; 30(8): 1232-43.
187. Merino D.M., McShane L.M., Fabrizio D. et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer.
188. Vokes N.I., Liu D., Ricciuti B. et al. Harmonization of Tumor Mutational Burden Quantificationand Association With Response to Immune Checkpoint Blockade in Non-Small-Cell Lung Cancer. JCO Precis Oncol 2019; 3:10.1200/PO.19.00171.
189. Rosenberg J.E., Hoffman-Censits J., Powles T. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387(10031): 909-20.
190. Chalmers Z.R., Connelly C.F., Fabrizio D. et al. Analysis of 100, 000 human cancer genomesreveals the landscape of tumor mutational burden. Genome Med 2017; 9(1): 34.
191. Kowanetz M., Zou W., Shames D.S. et al. Tumor mutation load assessed by FoundationOne (FM1) is associated with improved efficacy of atezolizumab (atezo) in patients with advanced NSCLC. Ann Oncol 2016; 27 (Suppl 6). doi: 10.1093/annonc/mdw363.25.
192. Ramalingam S., Hellmann M.D., Awad M.M. et al. Tumor mutational burden (TMB) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab + ipilimumab in first-line non-small cell lung cancer: identification of TMB cutoff from Checkmate 568. In AACR Annual Meeting 2018; Abstract #11317.
193. Balar A.V., Galsky M.D., Rosenberg J.E. et al. Atezolizumab as first-line treatment in cisplatinineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389(10064): 67 - 76.
194. Powles T.,  , van der Heijden M.S. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. In: 2018 Genitourinary Cancer Symposium. Lancet 2018; 391(10122): 748-57. doi: 10.1016/S0140-6736(17) 33297-X.
, van der Heijden M.S. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. In: 2018 Genitourinary Cancer Symposium. Lancet 2018; 391(10122): 748-57. doi: 10.1016/S0140-6736(17) 33297-X.
195. Dietel M., Bubendorf L., Dingemans A.M. et al. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax 2016; 71: 177.
196. Postmus P.E., Kerr K.M., Oudkerk M. et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017, 28 (Suppl. 4): iv1 - iv21.
197. Soffietti R., Abacioglu U., Baumert B. et al. Diagnosis and treatment of brain metastases fromsolid tumors: guidelines from the European Association of Neuro-Oncology (EANO). 2017; 19(2), 162-74.
198. De Wever W. Role of integrated PET/CT in the staging of non-small cell lung cancer. JBR-BTR2009; 92: 124.
199. Silvestri G.A., Gonzalez A.V., Jantz M.A. et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidencebased clinical practice guidelines. Chest 2013; 143(5) (Suppl): e211S - e250S. doi.org/10.1378/chest.12-2355.
200. National Lung Screening Trial Research Team; Aberle DR, Berg CD., Black W.C. et al. TheNational Lung Screening Trial: overview and study design. Radiology 2011; 258(1): 243-53. doi: 10.1148/radiol.10091808. Epub 2010 Nov 2.
201. National Lung Screening Trial Research Team, Church T.R., Black W.C., Aberle D.R. et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013; 368:1980. PMID: 23697514.
202. de Koning H.J., van der Aalst C.M., de Jong P.A. et al. Reduced Lung-Cancer Mortality withVolume CT Screening in a Randomized Trial. N Engl J Med 2020; 382(6): 503-13.
203. Sadate A., Occean B.V., Beregi J.P. et al. Systematic review and meta-analysis on the impact oflung cancer screening by low-dose computed tomography. Eur J Cancer 2020; 134: 107.
204. Herbst R.S., Tsuboi M., John T. et al. Osimertinib as adjuvant therapy in patients with stage IB-IIIA EGFR mutation-positive NSCLC after complete tumor resection: ADAURA. J Clin Oncol 2020; 38 (suppl 18):LBA5. doi: 10.1200/JCO.2020.38.18_suppl.LBA5.
205. Vokes E.E., Herndon J.E., Kelley M.J. et al. (). Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B.J Clin Oncol 2007; 25(13): 1698 - 1704.
206. Ahn J.S., Ahn Y.C., Kim J.H. et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol 2015; 33(24): 2660-6. doi:10.1200/JCO.2014.60.0130.
207. Ou S.H., Kwak E.L., Siwak-Tapp C. et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a nonsmall cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011; 6(5): 942-6.
208. Camidge R.D., Ou S.-H.I., Shapiro G. et al. Efficacy and safety of crizotinib in patients withadvanced c-MET-amplified non-small cell lung cancer. J Clin Oncol 2014; 32 (Suppl 5): abstr. 8001.
209. Li B.T., Shen R., Buonocore D. et al. Ado-Trastuzumab Emtansine for Patients With HER2Mutant Lung Cancers: Results From a Phase II Basket Trial. [published correction appears in J Clin Oncol 2019; 37(4):362]. J Clin Oncol. 2018; 36(24): 2532-7.
210. Hellmann M.D., Ciuleanu T.E., Pluzanski A. et al. Nivolumab plus Ipilimumab in Lung Cancerwith a High Tumor Mutational Burden. N Engl J Med 2018; 378(22): 2093-104.
211. Carbone D.P., Reck M., Paz-Ares L. et al. First-Line Nivolumab in Stage IV or Recurrent NonSmall-Cell Lung Cancer. N Engl J Med 2017; 376(25): 2415-426.
212. Drilon A., Wang L., Hasanovic A. et al. Response to Cabozantinib in patients with RET fusionpositive lung adenocarcinomas. Cancer Discov 2013; 3(6): 630-5.
213. Lee S.H., Lee J.K., Ahn M.J., et al. Vandetanib in pretreated patients with advanced non-smallcell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017; 28(2): 292-7.
214. Drilon A., Clark J.W., Weiss J. et al. Antitumor activity of crizotinib in lung cancers harboring aMET exon 14 alteration. Nat Med 2020; 26(1): 47 - 51.
215. Spigel D., De Marinis F., Giaccone G.et al. IMpower110: Interim OS Analysis of a Phase IIIStudy of Atezolizumab (atezo) vs Platinum-Based Chemotherapy (chemo) as 1L Treatment (tx) in PDL1-selected NSCLC//ESMO 2019 (Abs LBA78). Ann Oncol. 2019; 30 (suppl_5): v851 - v934. doi:10.1093/annonc/mdz394.
216. Herbst R.S., de Marinis F., Giaccone G. et al. Clinical efficacy of Atezolizumab (Atezo) in biomarker subgroups by SP142, SP263 and 22C3 PD-L1 immunochistochemistry (IHC) assys and by blood tumor mutational burden (BTMB): results from the IMPOWER110 study//ESMO IO 2019 (Abs LBA1).
217. Официальная инструкция по применению препарата. Доступно по: https://back-grls.pharmportal.ru/storage/instructions/%D0%9B%D0%9F004652/InstrImg_0001458248_0000609663/%D0%9B%D0%9F-004652[2020]_1.pdf.
218. Chou C.H., Hsu L.F. Model-based simulation to support the extended dosing regimens of atezolizumab. Eur J Clin Pharmacol 2020 Aug 17 [Online ahead of print]. doi: 10.1007/s00228-02002980-3. Epub ahead of print. PMID: 32808071.
219. Hellman M.D, Paz-Ares L., Caro R.B. et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell
Lung Cancer. N Engl J Med 2019; 381:2020-31.
220. Reck M., Ciuleanu T.-D., Dols M.C. et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles ofplatinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 2020; 38(15): 9501.
221. Lala M., Li T.R., de Alwis D.P., Sinha V. et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer 2020; 131: 68 - 75. doi: 10.1016/j.ejca.2020.02.016. Epub 2020 Apr 15. PMID: 32305010.
222. Grohe C., Gleiber W., Haas S. et al. Nintedanib plus docetaxel after progression on immunecheckpoint inhibitor therapy: insights from VARGADO, a prospective study in patients with lung adenocarcinoma. Future Oncol, Epub online:8 Jul 2019, doi: 10.2217/fon-2019-0262; 2019. p. 2699 - 2706.
223. Nakagawa K., Garon E., Seto T. et al. Ramucirumab plus erlotinib in patients with untreated, EGFR- mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(12): 1655-69.
224. Garon E., Ciuleanu T.-E., Arrieta O. et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384(9944): 665-73.
225. Stinchcombe T.E., J nne P.A., Wang X. et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol 2019; 5(10): 1448-55. doi.org/10.1001/jamaoncol.2019.1847.
226. Mok T., Camidge D.R., Gadgeel S.M. et al. Updated overall survival and final progression-freesurvival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020; 31(8): 1056-64. doi: 10.1016/j.annonc.2020.04.478. Epub 2020 May 11.
227. Paz-Ares L. Dvorkin M., Chen Y., Reinmuth N. Durvalumab ![]() tremelimumab + platinumetoposide in first-line extensive-stage SCLC (ES-SCLC): Updated results from the phase III CASPIAN study. J Clin Oncol 2020; 38: 15_suppl, 9002. doi: 10.1200/JCO.2020.38.15_suppl.9002.
tremelimumab + platinumetoposide in first-line extensive-stage SCLC (ES-SCLC): Updated results from the phase III CASPIAN study. J Clin Oncol 2020; 38: 15_suppl, 9002. doi: 10.1200/JCO.2020.38.15_suppl.9002.
228. Chen Y., Paz-Ares L., Dvorkin M. et al. First-line durvalumab plus platinum-etoposide in ESSCLC (CASPIAN): Impact of brain metastases on treatment patterns and outcomes. J ClinOncol 2020; 38: 15_suppl: 9068. doi: 10.1200/JCO.2020.38. 15_suppl. 9068.
229. Sesma A, Pardo J, Cruellas M, et al. From Tumor Mutational Burden to Blood T Cell Receptor: Looking for the Best Predictive Biomarker in Lung Cancer Treated with Immunotherapy. Cancers (Basel) 2020; 12.
230. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020; 21: 1353 - 1365.
231. Sholl LM, Hirsch FR, Hwang D, et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: A perspective from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol 2020; 15: 1409 - 1424
232. Yatabe Y, Dacic S, Borczuk AC, Warth A, Russell PA, Lantuejoul S, Beasley MB, Thunnissen E, Pelosi G, Rekhtman N, Bubendorf L, Mino-Kenudson M, Yoshida A, Geisinger KR, Noguchi M, Chirieac LR, Bolting J, Chung JH, Chou TY, Chen G, Poleri C, Lopez-Rios F, Papotti M, Sholl LM, Roden AC, Travis WD, Hirsch FR, Kerr KM, Tsao MS, Nicholson AG, Wistuba I and Moreira AL (2019). Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J Thorac Oncol 14(3): 377 - 407.
233. Schneider F, Beasley MB, Dacic S, Butnor KJ. Protocol for the Examination of Resection Specimens From Patients With Primary Non-Small Cell Carcinoma, Small Cell Carcinoma, or Carcinoid Tumor of the Lung. College of American Pathologists (CAP). 2021. - p. 1 - 22. www.cap.org/cancerprotocols.
234. International Collaboration on Cancer Reporting (ICCR). Lung Cancer Histopathology Reporting Guide. http://www.iccr-cancer.org/datasets (April 2023). - p. 1 - 33.
235. Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, Chou TY, Cooper W, Erasmus JJ, Ferreira CG, Goo JM, Heymach J, Hirsch FR, Horinouchi H, Kerr K, Kris M, Jain D, Kim YT, Lopez-Rios F, Lu S, Mitsudomi T, Moreira A, Motoi N, Nicholson AG, Oliveira R, Papotti M, Pastorino U, Paz-Ares L, Pelosi G, Poleri C, Provencio M, Roden AC, Scagliotti G, Swisher SG, Thunnissen E, Tsao MS, Vansteenkiste J, Weder W and Yatabe Y (2020). IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 15(5): 709 - 740.
236. Baskovich BW, Schneider F, Baras A, Birdsong GG, Fitzgibbons PL, Khoury JD, Seethala RR. Template for Reporting Results of Biomarker Testing of Specimens From Patients With Non-Small Cell Carcinoma of the Lung. College of American Pathologists (CAP). 2021. - p. 1 - 8. www.cap.org/cancerprotocols.
237. O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, Esteban E, Isla D, Martinez-Marti A, Faehling M, Tsuboi M, Lee JS, Nakagawa K, Yang J, Samkari A, Keller SM, Mauer M, Jha N, Stahel R, Besse B, Peters S; EORTC-1416-LCG/ETOP 8-15 - PEARLS/KEYNOTE-091 Investigators. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022 Oct; 23(10): 1274 - 1286. doi: 10.1016/S1470-2045(22) 00518-6.
238. Cascone T. CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II-IIIb NSCLC. Ann Oncol 2023; 34 (suppl_2): S1281 - S1282, Abstr.LBA1. https://doi.org/10.1016/j.annonc.2023.10.050
239. ALINA: Efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK + non-small cell lung cancer (NSCLC) B.J. Solomon, J.S. Ahn, R. Dziadziuszko, F. Barlesi, M. Nishio, D.H. Lee, J-S. Lee, W-Z. Zhong, H. Horinouchi, W. Mao, M.J. Hochmair, F. de Marinis, M.R. Migliorino, I. Bondarenko, T.O. Lohmann, T. Xu, A. Cardona Gavaldon, W. Bordogna, T. Ruf, Y-L. Wu. Annals of oncology VOLUME 34, SUPPLEMENT 2, S1295 - S1296, OCTOBER 2023 https://doi.org/10.1016/j.annonc.2023.10.051
240. Felip E, Altorki N, Zhou C, Cs szi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, Chen YM, Chella A, Sugawara S, Voong D, Wu F, Yi J, Deng Y, McCleland M, Bennett E, Gitlitz B, Wakelee H; IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021 Oct 9; 398(10308): 1344 - 1357. doi: 10.1016/S0140-6736(21) 02098-5.
241. Tagrisso demonstrated overwhelming efficacy benefit for patients with unresectable, stage III EGFR-mutated lung cancer in LAURA Phase III trial. News release. AstraZeneca. February 19, 2024. Accessed February 20, 2024. https://shorturl.at/gzEV1
242. Lee SM, Schulz C, Prabhash K, Kowalski D, Szczesna A, Han B, Rittmeyer A, Talbot T, Vicente D, Califano R, Cortinovis D, Le AT, Huang D, Liu G, Cappuzzo F, Reyes Contreras J, Reck M, Palmero R, Mak MP, Hu Y, Morris S, H glander E, Connors M, Biggane AM, Vollan HK, Peters S. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open- label, randomised controlled study. Lancet. 2023 Aug 5; 402(10400): 451 - 463. doi: 10.1016/S0140-6736(23) 00774-2
243. Robert M. Jotte, Marta Batus, Eric Bernicker et al., IMpower150: Exploratory efficacy analysis in patients (pts) with bulky disease.. JCO 38, e21637 - e21637(2020).
244. Nogami N, Barlesi F, Socinski MA et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J Thorac Oncol. 2022 Feb; 17(2): 309 - 323
245. Sezer A, Kilickap S, G m M, Bondarenko I, zg ro lu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, Clingan P, Sriuranpong V, Rizvi N, Gao B, Li S, Lee S, McGuire K, Chen CI, Makharadze T, Paydas S, Nechaeva M, Seebach F, Weinreich DM, Yancopoulos GD, Gullo G, Lowy I, Rietschel P. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021 Feb 13; 397(10274): 592 - 604. doi: 10.1016/S0140-6736(21) 00228-2. PMID: 33581821.
246. Reck M, Rodr guez-Abreu D, Robinson AG, Hui R, Cs szi T, F l p A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non- Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019 Mar 1; 37(7): 537 - 546. doi: 10.1200/JCO.18.00149. Epub 2019 Jan 8. PMID: 30620668.
247. IMpower110: Interim overall survival analysis of a phase III study of atezolizumab vs platinum-based chemotherapy as first-line treatment in PD-L1-selected NSCLC / D. Spigel, F. de Marinis, G. Giaccone [et al.]//Ann. Oncol. - 2019. - Vol. 30. - P. 915. - doi: 10.1093/annonc/mdz293.
248. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019; 7(5): 387 - 401.
249. Justin F. Gainor, MD, Director of the Center for Thoracic Cancers Program at Massachusetts General Hospital, Boston, was invited to discuss IMpower151 and ILLUMINATE at the 2023 World Conference on Lung Cancer.
250. Gadgeel S, Rodr guez-Abreu D, Speranza G, Esteban E, Felip E, D mine M, Hui R, Hochmair MJ, Clingan P, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Garon EB, Novello S, Rubio-Viqueira B, Boyer M, Kurata T, Gray JE, Yang J, Bas T, Pietanza MC, Garassino MC. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2020 May 10; 38(14): 1505 - 1517. doi: 10.1200/JCO.19.03136. Epub 2020 Mar 9. PMID: 32150489.
251. Novello S, Kowalski DM, Luft A, G m M, Vicente D, Mazi res J, Rodr guez-Cid J, Tafreshi A, Cheng Y, Lee KH, Golf A, Sugawara S, Robinson AG, Halmos B, Jensen E, Schwarzenberger P, Pietanza MC, Paz-Ares L. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol. 2023 Apr 10; 41(11): 1999 - 2006. doi: 10.1200/JCO.22.01990. Epub 2023 Feb 3. PMID: 36735893; PMCID: PMC10082300.
252. Zhou C, Hu Y, Arkania E, Kilickap S, Ying K, Xu F, Wu L, Wang X, Viguro M, Makharadze T, Sun H, Luo F, Shi J, Zang A, Pan Y, Chen Z, Jia Z, Kuchava V, Lu P, Zhang L, Cheng Y, Kang W, Wang Q, Yu H, Li J, Zhu J; ASTRUM-004 Investigators. A global phase 3 study of serplulimab plus chemotherapy as first-line treatment for advanced squamous non-small-cell lung cancer (ASTRUM-004). Cancer Cell. 2023 Dec 21: S1535 - 6108(23) 00432-4. doi: 10.1016/j.ccell.2023.12.004. Epub ahead of print. PMID: 38181795.
253. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1/S.S. Ramalingam, T.E. Ciuleanu, A. Pluzanski [et al.]//J. Clin. Oncol. - 2020. - Vol. 38, Suppl. - Abstr. 9500.
254. Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F, Goto Y, Kim DW, Wu YL, Jassem J, L pez FL, Soo RA, Shaw AT, Polli A, Messina R, Iadeluca L, Toffalorio F, Felip E. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023 Apr; 11(4): 354 - 366. doi: 10.1016/S2213-2600(22) 00437-4. Epub 2022 Dec 16. PMID: 36535300.
255. Wolf J., Seto T., Han J.-Y., Reguart N., Garon E.B., Groen H.J.M., Tan D.S.W., Hida T., de Jonge M., Orlov S.V., et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020; 383: 944 - 957.
256. Drilon A, Chiu CH, Fan Y, Cho BC, Lu S, Ahn MJ, Krebs MG, Liu SV, John T, Otterson GA, Tan DSW, Patil T, Dziadziuszko R, Massarelli E, Seto T, Doebele RC, Pitcher B, Kurtsikidze N, Heinzmann S, Siena S. Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion-Positive NSCLC. JTO Clin Res Rep. 2022 Apr 29; 3(6): 100332. doi: 10.1016/j.jtocrr.2022.100332. PMID: 35663414; PMCID: PMC9160474.
257. Dy GK, Govindan R, Velcheti V, Falchook GS, Italiano A, Wolf J, Sacher AG, Takahashi T, Ramalingam SS, Dooms C, Kim DW, Addeo A, Desai J, Schuler M, Tomasini P, Hong DS, Lito P, Tran Q, Jones S, Anderson A, Hindoyan A, Snyder W, Skoulidis F, Li BT. Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non-Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100. J Clin Oncol. 2023 Jun 20; 41(18): 3311 - 3317. doi: 10.1200/JCO.22.02524. Epub 2023 Apr 25. PMID: 37098232; PMCID: PMC10414711.
258. Hong DS, Bauer TM, Lee JJ, Dowlati A, Brose MS, Farago AF, Taylor M, Shaw AT, Montez S, Meric-Bernstam F, Smith S, Tuch BB, Ebata K, Cruickshank S, Cox MC, Burris HA 3rd, Doebele RC. Larotrectinib in adult patients with solid tumours: a multi-centre, open-label, phase I dose-escalation study. Ann Oncol. 2019 Feb 1; 30(2): 325 - 331. doi: 10.1093/annonc/mdy539. PMID: 30624546; PMCID: PMC6386027.
259. Goto K., Sang-We K., Kubo T., Goto Y., Ahn M.-J., Planchard D., Kim D.-W., Yang J.-H., Yang T.-Y., Pereira K., et al. LBA55 Trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-mutant metastatic non-small cell lung cancer (NSCLC): Interim results from the phase 2 DESTINY-Lung02 trial. Ann. Oncol. 2022; 33: S1422. doi: 10.1016/j.annonc.2022.08.057.
260. Drilon A, Subbiah V, Gautschi O, Tomasini P, de Braud F, Solomon BJ, Shao-Weng Tan D, Alonso G, Wolf J, Park K, Goto K, Soldatenkova V, Szymczak S, Barker SS, Puri T, Bence Lin A, Loong H, Besse B. Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J Clin Oncol. 2023 Jan 10; 41(2): 385 - 394. doi: 10.1200/JCO.22.00393. Epub 2022 Sep 19. Erratum in: J Clin Oncol. 2023 Nov 1; 41(31): 4941. PMID: 36122315; PMCID: PMC9839260.
261. B.C. Cho, C.-H. Chiu, E. Massarelli, G.L. Buchschacher Jr, K. Goto, T.R. Overbeck, H.H.F. Loong, C. Chee, P. Garrido, S. Heinzmann, W. Bordogna, H. Zeuner, S. Osborne, T. John Updated efficacy and safety of entrectinib in patients (pts) with locally advanced/metastatic NTRK fusion-positive (fp) non- small cell lung cancer (NSCLC) J. Clin. Oncol., 41 (2023), p. 9047
262. Smit EF, Felip E, Uprety D, et al. Trastuzumab deruxtecan in patients with HER2-overexpressing metastatic non-small cell lung cancer: results from the DESTINY-Lung01 trial [Стендовый доклад представлен на ежегодном заседании Европейского общества медицинской онкологии (ESMO); 9 - 13 сентября 2022 г., Париж, Франция. Стендовый доклад 975P и дополнение
263. Nakagawa K., Nagasaka M., Felip E., Pacheco J., Baik C., Goto Y., Saltos A., Li B., Udagawa H., Gadgeel S., et al. OA04.05 Trastuzumab Deruxtecan in HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Interim Results of DESTINY-Lung01. J. Thorac. Oncol. 2021; 16: S109 - S110. doi: 10.1016/j.jtho.2021.01.285
264. Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC, Hensing TA, Socinski MA. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004 Dec 1; 22(23): 4837-45. doi: 10.1200/JCO.2004.01.178. Erratum in: J Clin Oncol. 2005 Jan 1; 23(1): 248. PMID: 15570087.
265. Spiro SG, James LE, Rudd RM, Trask CW, Tobias JS, Snee M, Gilligan D, Murray PA, Ruiz de Elvira MC, O'Donnell KM, Gower NH, Harper PG, Hackshaw AK; London Lung Cancer Group. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006 Aug 20; 24(24): 3823-30. doi: 10.1200/JCO.2005.05.3181. PMID: 16921033.
266. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an openlabel, phase 3, randomised, superiority trial. Lancet Oncol 2017; 18: 1116 - 1125.
267. Bogart JA, Wang XF, Masters GA, et al. High-dose once-daily thoracic radiotherapy in limited-stage small-cell lung cancer: CALGB 30610 (Alliance)/RTOG 0538. J Clin Oncol 2023; 41: 2394 - 2402.
268. Ganti A, Dueck AC, Fruth B, et al. Comparison of quality of life in patients randomized to highdose once daily (QD) thoracic radiotherapy (TRT) with standard twice daily (BID) TRT in limited stage small cell lung cancer (LS-SCLC) on CALGB 30610 (Alliance, Sub-study CALGB 70702) [abstract]. J Clin Oncol 2022; 40: 8504.
269.  , Killingberg KT,
, Killingberg KT,  , et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet Oncol 2021; 22: 321 - 331.
, et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet Oncol 2021; 22: 321 - 331.
270. Qiu B, Li QW, Liu JL, et al. Moderately hypofractionated once-daily compared with twice-daily thoracic radiation therapy concurrently with etoposide and cisplatin in limited-stage small-cell lung cancer: a multi- center, Phase II, randomized trial. Int J Radiat Oncol Biol Phys 2021; 111: 424 - 435.
271. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 1999; 17: 2092 - 2099.
272. Yee D, Butts C, Reiman A, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol 2012; 102: 234 - 238.
273. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015; 385: 36 - 42.
274. Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer-Authors' reply. Lancet 2015; 385: 1292 - 1293.
275.  , Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009; 10: 467 - 474.
, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009; 10: 467 - 474.
276. Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limiteddisease small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 81: 77 - 84.
277.  , Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with smallcell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999; 341: 476 - 484.
, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with smallcell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999; 341: 476 - 484.
278. Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. The Early Clinical Trials Group of the EORTC. Eur J Cancer 1994; 30A: 1058-60
279. Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, Salvagni S, Taylor M, Amin A, Camidge DR, Horn L, Calvo E, Li A, Lin WH, Callahan MK, Spigel DR. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol. 2020 Mar; 15(3): 426 - 435. doi: 10.1016/j.jtho.2019.10.004. Epub 2019 Oct 17. PMID: 31629915.
280. Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr, Delord JP, Gao B, Planchard D, Gottfried M, Zer A, Jalal SI, Penel N, Mehnert JM, Matos I, Bennouna J, Kim DW, Xu L, Krishnan S, Norwood K, Ott PA. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol. 2020 Apr; 15(4): 618 - 627. doi: 10.1016/j.jtho.2019.12.109. Epub 2019 Dec 20. PMID: 31870883.
281. Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: Clinical Nutrition in cancer.//Clin Nutr. 2021; 40(5): 2898 - 2913. doi: 10.1016/j.clnu.2021.02.005
282. Lugg ST, Tikka T, Agostini PJ, Kerr A, et al. Smoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgery.//J Cardiothorac Surg. 2017; 12(1): 52. doi: 10.1186/s13019-017-0614-4.
283. Caini S, Del Riccio M, Vettori V, et al. Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: a systematic review and meta-snalysis.//J Thorac Oncol. 2022; 17(5): 623 - 636. doi: 10.1016/j.jtho.2021.12.005.
284. Yang J, Zhang Q, Wang X. Role of nutritional support for postoperative recovery of respiratory function in patients with primary lung cancer.//Oncol Lett. 2018; 16(5): 5978 - 5982. doi: 10.3892/ol.2018.9348
285. Wislez M, Mazieres J, Lavole A, Zalcman G, Carre O, Egenod T, Caliandro R, Dubos-Arvis C, Jeannin G, Molinier O, Massiani MA, Langlais A, Morin F, Le Pimpec Barthes F, Brouchet L, Assouad J, Milleron B, Damotte D, Antoine M, Westeel V. Neoadjuvant durvalumab for resectable non-small-cell lung cancer (NSCLC): results from a multicenter study (IFCT-1601 IONESCO). J Immunother Cancer. 2022 Oct; 10(10): e005636. doi: 10.1136/jitc-2022-005636.
286. Burotto M, Zvirbule Z, Mochalova A. et al. IMscin001 Part 2: a randomised phase III, open-label, multicentre study examining the pharmacokinetics, efficacy, immunogenicity, and safety of atezolizumab subcutaneous versus intravenous administration in previously treated locally advanced or metastatic non- small-cell lung cancer and pharmacokinetics comparison with other approved indications. Ann Oncol. 2023 Aug; 34(8): 693 - 702
287. Laktionov K.K. MVM, Smolin A.V., Dvorkin M.V., Andabekov T.T., Kozlov V.V., Odintsova S.V., Dvoretsky S.Yu., Fadeeva N. .V., Musaev G.Kh., Udovitsa D.P., Pirmagomedov A.Sh., Poddubskaya E.V., Mochalova A.S., Varvyanskaya N.V., Khasanova A.I., Semiglazova T.Yu., Kislov N.V., Shumskaya I.S., Narimanov M.N., Tarasova A.V., Gladkov O.A., Sorokina I.V., Zinkina-Orikhan A.V., Linkova Yu.N., Kryukov F.B. Efficacy and safety of prolgolimab in the 1st line of combination therapy for non-squamous NSCLC according to the results of the phase 3 clinical trial BCD-100-3/DOMAJOR. in the collection of the XXVII Russian Oncological Congress - Abstracts of poster reports and accepted for publication, series Journal "Malignant Tumors" 2023 (Special issue): 131-2.
288. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G, Hussein M, Lim FL, Yang CT, Araujo LH, Saito H, Reinmuth N, Shi X, Poole L, Peters S, Garon EB, Mok T; POSEIDON investigators. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol. 2023 Feb 20; 41(6): 1213 - 1227. doi: 10.1200/JCO.22.00975. Epub 2022 Nov 3. PMID: 36327426; PMCID: PMC9937097.
289. Planchard D, J nne PA, Cheng Y, Yang JC, Yanagitani N, Kim SW, Sugawara S, Yu Y, Fan Y, Geater SL, Laktionov K, Lee CK, Valdiviezo N, Ahmed S, Maurel JM, Andrasina I, Goldman J, Ghiorghiu D, Rukazenkov Y, Todd A, Kobayashi K; FLAURA2 Investigators. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med. 2023 Nov 23; 389(21): 1935 - 1948. doi: 10.1056/NEJMoa2306434. Epub 2023 Nov 8. PMID: 37937763.
290. Заридзе Д.Г., Мукерия А.Ф., Шаньгина О.В., Стилиди И.С. Отказ от курения после постановки диагноза рака легкого улучшает прогноз заболевания. Злокачественные опухоли. 2021; 11(3): 15 - 22. https://doi.org/10.18027/2224-5057-2021-11-3-15-22
291. Hong H. et al. Pleural recurrence after transthoracic needle lung biopsy in stage I lung cancer: a systematic review and individual patient-level meta-analysis.//Thorax. 2021 Jun; 76(6): 582 - 590. doi:10.1136/thoraxjnl-2020-216492.
292. Hao M. et al. Effects of preoperative needle biopsy for lung cancer on survival and recurrence: a systematic review and meta-analysis.//Surg Today. 2024 Feb; 54(2): 95 - 105. doi: 10.1007/s00595-022-02617-1.
293. Пилюс П.С., Дрокин И.С., Баженова Д.А., Маковская Л.А., Синицын В.Е. Оценка перспектив использования технологий искусственного интеллекта для анализа КТ изображений органов грудной клетки с целью выявления признаков злокачественных новообразований в легких. Медицинская визуализация. 2023; 27(2): 138 - 146. https://doi.org/10.24835/1607-0763-1151
294. Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T. Survival of patients with resected N 2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000 Aug; 18(16): 2981-9. doi: 10.1200/JCO.2000.18.16.2981. PMID: 10944131.
295. Belani CP, Lee JS, Socinski MA, Robert F, Waterhouse D, Rowland K, Ansari R, Lilenbaum R, Natale RB. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005 Jul; 16(7): 1069-75. doi: 10.1093/annonc/mdi216. Epub 2005 Apr 28. PMID: 15860487.
296. Kosmidis P, Mylonakis N, Skarlos D, Samantas E, Dimopoulos M, Papadimitriou C, Kalophonos C, Pavlidis N, Nikolaidis C, Papaconstantinou C, Fountzilas G. Paclitaxel (175 mg/m2) plus carboplatin (6 AUC) versus paclitaxel (225 mg/m2) plus carboplatin (6 AUC) in advanced non-small-cell lung cancer (NSCLC): a multicenter randomized trial. Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol. 2000 Jul; 11(7): 799 - 805. doi: 10.1023/a:1008389402580. PMID: 10997806.
297. Waits TM, Johnson DH, Hainsworth JD, Hande KR, Thomas M, Greco FA. Prolonged administration of oral etoposide in non-small-cell lung cancer: a phase II trial. J Clin Oncol. 1992 Feb; 10(2): 292-6. doi: 10.1200/JCO.1992.10.2.292. PMID: 1310104.
298. CheckMate 078 - Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu HY, Wu L, Feng J, Zhang Y, Luft AV, Zhou J, Ma Z, Lu Y, Hu C, Shi Y, Baudelet C, Cai J, Chang J. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol. 2019 May; 14(5): 867 - 875. doi: 10.1016/j.jtho.2019.01.006. Epub 2019 Jan 17. PMID: 30659987.
299. KEYNOTE-010 - Herbst RS, Baas P, Kim DW, Felip E,  , Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016 Apr 9; 387(10027): 1540 - 1550. doi: 10.1016/S0140-6736(15) 01281-7. Epub 2015 Dec 19. PMID: 26712084.
, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016 Apr 9; 387(10027): 1540 - 1550. doi: 10.1016/S0140-6736(15) 01281-7. Epub 2015 Dec 19. PMID: 26712084.
300. J nger ST, Reinecke D, Meissner AK, Goldbrunner R, Grau S. Resection of symptomatic non-small cell lung cancer brain metastasis in the setting of multiple brain metastases. J Neurosurg. 2021 Oct 29; 136(6): 1576 - 1582. doi: 10.3171/2021.7.JNS211172. PMID: 34715653.
301. Soffietti, R.,  , R. & Mutani, R. Management of brain metastases. J Neurol 249, 1357 - 1369 (2002). https://doi.org/10.1007/s00415-002-0870-6
, R. & Mutani, R. Management of brain metastases. J Neurol 249, 1357 - 1369 (2002). https://doi.org/10.1007/s00415-002-0870-6
302. Khan M, Zhao Z, Arooj S, Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer. 2021 Feb 16; 21(1): 167. doi: 10.1186/s12885-021-07889-3. PMID: 33593308; PMCID: PMC7885379.
303. Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T, Saijo N. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007 Jul 16; 97(2): 162-9. doi: 10.1038/sj.bjc.6603810. Epub 2007 Jun 19. PMID: 17579629; PMCID: PMC2360311.
304. Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T, Saijo N. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007 Jul 16; 97(2): 162-9. doi: 10.1038/sj.bjc.6603810. Epub 2007 Jun 19. PMID: 17579629; PMCID: PMC2360311.
305. Schmittel A, Fischer von Weikersthal L, Sebastian M, et al. A randomized phase II trial of irinotecan plus carboplatin versus etoposide plus carboplatin treatment in patients with extended disease small-cell lung cancer. Ann Oncol 2006; 17: 663 - 667.
306. Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85 - 91.
307. Hanna N, Bunn Jr, PA, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive stage disease small-cell lung cancer. J Clin Oncol 2006; 24: 2038 - 2043.
308. Einhorn LH, Pennington K, McClean J. Phase II trial of daily oral VP-16 in refractory small cell lung cancer: a Hoosier Oncology Group study. Semin Oncol 1990; 17: 32 - 35.
309. Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol 1990; 8: 1613 - 1617.
310. Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol 2003; 21: 1550 - 1555.
311. Van der Lee I, Smit EF, van Putten JW, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol 2001; 12: 557 - 561.
312. Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014; 86: 237 - 240.
313. Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012; 18: 1138 - 1145.
314. Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Gao D, Serizawa T, Yomo S, Aiyama H, Higuchi Y, Shuto T, Akabane A, Sato Y, Niranjan A, Faramand AM, Lunsford LD, McInerney J, Tuanquin LC, Zacharia BE, Chiang V, Singh C, Yu JB, Braunstein S, Mathieu D, Touchette CJ, Lee CC, Yang HC, Aizer AA, Cagney DN, Chan MD, Kondziolka D, Bernstein K, Silverman JS, Grills IS, Siddiqui ZA, Yuan JC, Sheehan JP, Cordeiro D, Nosaki K, Seto T, Deibert CP, Verma V, Day S, Halasz LM, Warnick RE, Trifiletti DM, Palmer JD, Attia A, Li B, Cifarelli CP, Brown PD, Vargo JA, Combs SE, Kessel KA, Rieken S, Patel S, Guckenberger M, Andratschke N, Kavanagh BD, Robin TP. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE- SCLC Cohort Study. JAMA Oncol. 2020 Jul 1; 6(7): 1028 - 1037. doi: 10.1001/jamaoncol.2020.1271. Erratum in: JAMA Oncol. 2020 Sep 1; 6(9): 1473. doi: 10.1001/jamaoncol.2020.3404. PMID: 32496550; PMCID: PMC7273318.
315. Park H, Park J, Woo SY, Yi YH, Kim K. Effect of high-frequency chest wall oscillation on pulmonary function after pulmonary lobectomy for non-small cell lung cancer. Crit Care Med. 2012 Sep; 40(9): 2583-9. doi: 10.1097/CCM.0b013e318258fd6d. PMID: 22732281.
316. Agostini P, Naidu B, Cieslik H, Steyn R, Rajesh PB, Bishay E, Kalkat MS, Singh S. Effectiveness of incentive spirometry in patients following thoracotomy and lung resection including those at high risk for developing pulmonary complications. Thorax. 2013 Jun; 68(6): 580-5. doi: 10.1136/thoraxjnl-2012-202785. Epub 2013 Feb 21. PMID: 23429831.
317. De Ruysscher D, van Loon J. Radical Radiotherapy for Locally Advanced Non-small Cell Lung Cancer: When Should Concurrent Chemoradiotherapy Not Be Used? Clin Oncol (R Coll Radiol). 2016 Nov; 28(11): 708 - 711. doi: 10.1016/j.clon.2016.07.011. Epub 2016 Aug 9. PMID: 27519158.
318. Videtec GMM, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017; 7: 295 - 301
319. Ball D, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 nonsmall-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019; 20: 494 - 503
320. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992 Jun; 10(6): 890-5. doi: 10.1200/JCO.1992.10.6.890. PMID: 1316951.
321. Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992 Dec 3; 327(23): 1618-24. doi: 10.1056/NEJM199212033272302. PMID: 1331787.
322. De Ruysscher D, Lueza B, Le P choux C, Johnson DH, O'Brien M, Murray N, Spiro S, Wang X, Takada M, Lebeau B, Blackstock W, Skarlos D, Baas P, Choy H, Price A, Seymour L, Arriagada R, Pignon JP; RTT-SCLC Collaborative Group. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. 2016 Oct; 27(10): 1818-28. doi: 10.1093/annonc/mdw263. Epub 2016 Jul 19. PMID: 27436850; PMCID: PMC5035783.
323. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden S, Le Pechoux C, McMenemin R, Mohammed N, O'Brien M, Pantarotto J, Surmont V, Van Meerbeeck JP, Woll PJ, Lorigan P, Blackhall F; CONVERT Study Team. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017 Aug; 18(8): 1116 - 1125. doi: 10.1016/S1470-2045(17) 30318-2. Epub 2017 Jun 20. PMID: 28642008; PMCID: PMC5555437.
324. Zhang C, Zhao G, Wu H, Jiang J, Duan W, Fan Z, Wang Z, Wang R. Application of postoperative adjuvant radiotherapy in limited-stage small cell lung cancer: A systematic review and meta-analysis. Radiother Oncol. 2024 Apr; 193: 110123. doi: 10.1016/j.radonc.2024.110123. Epub 2024 Feb 2. PMID: 38309584.
325. Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011 Apr 1; 79(5): 1487-95. doi: 10.1016/j.ijrobp.2009.12.061. Erratum in: Int J Radiat Oncol Biol Phys. 2012 Sep 1; 84(1): 6. Grewal, Jai [added]. PMID: 20399573; PMCID: PMC2908725.
326. Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D. Treatment for Brain Metastases: ASCO-SNO- ASTRO Guideline. J Clin Oncol. 2022 Feb 10; 40(5): 492 - 516. doi: 10.1200/JCO.21.02314. Epub 2021 Dec 21. Erratum in: J Clin Oncol. 2022 Apr 20; 40(12): 1392. doi: 10.1200/JCO.22.00593. PMID: 34932393.
327. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990 Feb 22; 322(8): 494 - 500. doi: 10.1056/NEJM199002223220802. PMID: 2405271.
328. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993 Jun; 33(6): 583-90. doi: 10.1002/ana.410330605. PMID: 8498838.
329. Loganadane G, Hendriks L, Le P choux C, Levy A. The Current Role of Whole Brain Radiation Therapy in Non-Small Cell Lung Cancer Patients. J Thorac Oncol. 2017 Oct; 12(10): 1467 - 1477. doi: 10.1016/j.jtho.2017.07.006. Epub 2017 Jul 19. PMID: 28733269.
330. Planchard D, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF (V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017; 18: 1307 - 1316
331. Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, Rigas JR, Upalawanna A, D'Amelio AM Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously treated BRAF (V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016 Jul; 17(7): 984 - 993. doi: 10.1016/S1470-2045(16) 30146-2. Epub 2016 Jun 6. PMID: 27283860; PMCID: PMC4993103.
332. Drilon A, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1 - 2 trials. Lancet Oncol 2020; 21: 261 - 270.
333. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodr guez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018 Jun 14; 378(24): 2288 - 2301. doi: 10.1056/NEJMoa1716948. Epub 2018 Jun 4. PMID: 29863955.
334. Cascone T, Awad M, Spicer J, et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med 2024; 390: 1756 - 1769.
335. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022; 386: 1973 - 1985
336. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 2023; 389: 491 - 503
337. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med 2023; 389: 1672 - 1684
338.  , Chouaid C,
, Chouaid C,  , et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30: 3516 - 3524
, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30: 3516 - 3524
339. Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003; 21:3016-30
340. Kreuter M, Vansteenkiste J, Fishcer JR, et al. Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Ann Oncol 2013; 24: 986 - 992.
341. Strauss GM, Herndon III JE, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008; 26: 5043 - 5051.
342. Usami N, Yokoi K, Hasegawa Y, et al. Phase II study of carboplatin and gemcitabine as adjuvant chemotherapy in patients with completely resected non-small cell lung cancer: a report from the Central Japan Lung Study Group, CJLSG 0503 trial. Int J Clin Oncol 2010; 15: 583 - 587.
343. Zhang L, Ou W, Liu Q, et al. Pemetrexed plus carboplatin as adjuvant chemotherapy in patients with curative resected non-squamous non-small cell lung cancer. Thorac Cancer 2014; 5: 50 - 56.
344. Jeffrey D Bradley, Rebecca Paulus, Ritsuko Komak. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015 Feb; 16(2):187-99.
345. Lu S, Kato T, Dong X, et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N Engl J Med 2024; 391: 585 - 597.
346. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22: 198 - 211
347. NCCN Guidelines Version 7.2025. Non-Small Cell Lung Cancer. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
348. Wei Ou, Hai-bo Sun, Xiong Ye et. al. Adjuvant Carboplatin-based Chemotherapy in Resected Stage IIIA-N2 Non-small Cell Lung Cancer. Journal of Thoracic Oncology 2010; V. 5: 1033 - 1041.
349. Patrick M. Forde, Jonathan D. Spicer, Mariano Provencio et. al. Overall Survival with Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer. New England Journal of Medicine. 2025; 393: 741 - 752.
350. Jassem J. et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC)//Journal of Clinical Oncology. - 2010. - Т. 28. - N. 15_suppl. - С. 7500 - 7500.
351. Gridelli С., Chen T., Ko A. et al. Paclitaxel/carboplatin in elderly patients with advanced squamous non-small cell lung cancer: a retrospective analysis of a Phase III trial. Drug Des Devel Ther 2018; 12:1445-51. doi: 10.2147/DDDT.S155750.
352. Ratanatharathorn V. et al. Phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer//Lung Cancer. - 2001. - Т. 31. - N. 2-3. - С. 257-265.
353. Petr Zatloukal, lubos Petruzelka, Milada Zemanova et. al. Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: a phase III randomized trial. Lung Cancer.2003; 321 - 331
354. Spigel D. R. et al. A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer//Journal of Thoracic Oncology. - 2012. - Т. 7. - N. 1. - С. 196 - 202.
355. Kang D. H. et al. Efficacy of vinorelbine monotherapy as third-or further-line therapy in patients with advanced non-small-cell lung cancer//Oncology. - 2019. - Т. 97. - N. 6. - С. 356 - 364.
356. Nobili S. et al. Vinorelbine in non-small cell lung cancer: real-world data from a single-institution experience//Oncology Research. - 2020. - Т. 28. - N. 3. - С.
357. Xu K. et al. The efficacy and toxicity of metronomic oral vinorelbine monotherapy in patients with non-small cell lung cancer: a meta-analysis//International Journal of Clinical Oncology. - 2020. - Т. 25. - N. 9. - С. 1624 - 1634.
358. Ricci S. et al. Gemcitabine monotherapy in elderly patients with advanced non-small cell lung cancer: a multicenter phase II study//Lung cancer. - 2000. - Т. 27. - N. 2. - С. 75 - 80.
359. Garassino M. C. et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study//Journal of Clinical Oncology. - 2023. - Т. 41. - N. 11. - С. 1992 - 1998.
360. Paz-Ares L. G. et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). - 2018.
361. R Rosell, U Gatzemeier, D C Betticher et.al. Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non-small-cell lung cancer: a cooperative multinational trial. Ann Oncol. 2002; 13(10):1539-49.
362.  , S F Powell, M J Hochmair et.al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021; 32(7): 881 - 895
, S F Powell, M J Hochmair et.al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021; 32(7): 881 - 895
363. Sankar K., Gadgeel S. M., Qin A. Molecular therapeutic targets in non-small cell lung cancer //Expert review of anticancer therapy. - 2020. - Т. 20. - N. 8. - С. 647 - 661.
364. Guang-Jian Yang, Jun Li, Hai-Yan Xu et.al. Osimertinib for Chinese advanced non-small cell lung cancer patients harboring diverse EGFR exon 20 insertion mutations. Lung Cancer. 2021 Feb:152:39-48.
365. Keunchil Park, Chong-Jen Yu, Sang-We Kim et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol.2016; 2; (3): 305 - 312.
366. Helena A Yu, Camelia S Sima, James Huang et.al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013; 8(3):346-51.
367. David Planchard, Michael J. Boyer, Jong-Seok Lee et.al. Postprogression Outcomes for Osimertinib versus Standard-of-Care EGFR-TKI in Patients with Previously Untreated EGFR-mutated Advanced Non-Small Cell Lung Cancer. Clin Cancer Res. 2019; 25(7): 2058 - 2063.
368. Benjamin J. Solomon, Geoffrey Liu, Enriqueta Felip et. al. Lorlatinib Versus Crizotinib in Patients with Advanced ALK -Positive Non-Small Cell Lung Cancer: 5-Year Outcomes from the Phase III CROWN Study. Journal of Clinical Oncology. 2024; 42(29): 3400 - 3409
369. Wu YL, Smit EF, Bauer TM. Capmatinib for patients with non-small cell lung cancer with MET exon 14 skipping mutations: A review of preclinical and clinical studies. Cancer Treat Rev. 2021 Apr; 95:102173. doi: 10.1016/j.ctrv.2021.102173. Epub 2021 Mar 1. PMID: 33740553.
370. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020 Feb; 21(2):271-282. doi: 10.1016/S1470-2045(19) 30691-6. Epub 2019 Dec 11. Erratum in: Lancet Oncol. 2020 Feb; 21(2):e70. doi: 10.1016/S1470-2045(20) 30029-2. Erratum in: Lancet Oncol. 2020 Jul; 21(7):e341. doi: 10.1016/S1470- 2045(20) 30345-4. Erratum in: Lancet Oncol. 2020 Aug; 21(8):e372. doi: 10.1016/S1470-2045(20) 30382-X. Erratum in: Lancet Oncol. 2021 Oct; 22(10):e428. doi: 10.1016/S1470-2045(21) 00538-6. PMID: 31838007; PMCID: PMC7461630.
371. Tjong MC, Mak RH, Louie AV. Trastuzumab Deruxtecan in Non-Small-Cell Lung Cancer. N Engl J Med. 2022 May 5; 386(18):1770. doi: 10.1056/NEJMc2202305. PMID: 35507495.
372. Zhou C, Solomon B, Loong HH, Park K,  , Arriola E, Novello S, Han B, Zhou J, Ardizzoni A, Mak MP, Santini FC, Elamin YY, Drilon A, Wolf J, Payakachat N, Uh MK, Rajakumar D, Han H, Puri T, Soldatenkova V, Lin AB, Lin BK, Goto K; LIBRETTO-431 Trial Investigators. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N Engl J Med. 2023 Nov 16; 389(20): 1839 - 1850. doi: 10.1056/NEJMoa2309457. Epub 2023 Oct 21. PMID: 37870973; PMCID: PMC10698285.
, Arriola E, Novello S, Han B, Zhou J, Ardizzoni A, Mak MP, Santini FC, Elamin YY, Drilon A, Wolf J, Payakachat N, Uh MK, Rajakumar D, Han H, Puri T, Soldatenkova V, Lin AB, Lin BK, Goto K; LIBRETTO-431 Trial Investigators. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N Engl J Med. 2023 Nov 16; 389(20): 1839 - 1850. doi: 10.1056/NEJMoa2309457. Epub 2023 Oct 21. PMID: 37870973; PMCID: PMC10698285.
373. Mansfield AS, Herbst RS, de Castro G Jr, Hui R, Peled N, Kim DW, Novello S, Satouchi M, Wu YL, Garon EB, Reck M, Robinson AG, Samkari A, Piperdi B, Ebiana V, Lin J, Mok TSK. Outcomes With Pembrolizumab Monotherapy in Patients With Programmed Death-Ligand 1-Positive NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin Res Rep. 2021 Jul 1; 2(8):100205. doi: 10.1016/j.jtocrr.2021.100205. PMID: 34590048; PMCID: PMC8474394.
374. Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346: 85-91
375. Okamoto H, Watanabe K, Nishiwaki Y, Mori K, Kurita Y, Hayashi I, Masutani M, Nakata K, Tsuchiya S, Isobe H, Saijo N. Phase II study of area under the plasma concentration-versus-time curve-based carboplatin plus standard-dose intravenous etoposide in elderly patients with small-cell lung cancer. J Clin Oncol. 1999 Nov; 17(11):3540-5. doi: 10.1200/JCO.1999.17.11.3540. PMID: 10550152.
376. Ihde DC, Mulshine JL, Kramer BS, Steinberg SM, Linnoila RI, Gazdar AF, Edison M, Phelps RM, Lesar M, Phares JC, et al. Prospective randomized comparison of high-dose and standard-dose etoposide and cisplatin chemotherapy in patients with extensive-stage small-cell lung cancer. J Clin Oncol. 1994 Oct; 12(10):2022-34. doi: 10.1200/JCO.1994.12.10.2022. PMID: 7931470.
377. Sambrook RJ, Girling DJ. A national survey of the chemotherapy regimens used to treat small cell lung cancer (SCLC) in the United Kingdom. Br J Cancer. 2001 Jun 1; 84(11):1447-52. doi: 10.1054/bjoc.2001.1817. PMID: 11384091; PMCID: PMC2363653.
378. Girling DJ. Comparison of oral etoposide and standard intravenous multidrug chemotherapy for small-cell lung cancer: a stopped multicentre randomised trial. Medical Research Council Lung Cancer Working Party. Lancet. 1996 Aug 31; 348(9027):563-6. doi: 10.1016/s0140-6736(96) 02005-3. PMID: 8774567.
379. Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, Rakovitch E, Chow E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018 Jan 25; 1(1):CD003869. doi: 10.1002/14651858.CD003869.pub4. PMID: 29365347; PMCID: PMC6491334.
380. Negoro S, Fukuoka M, Masuda N, Takada M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Niitani H, Taguchi T. Phase I study of weekly intravenous infusions of CPT-11, a new derivative of camptothecin, in the treatment of advanced non-small-cell lung cancer. J Natl Cancer Inst. 1991 Aug 21; 83(16):1164-8. doi: 10.1093/jnci/83.16.1164. PMID: 1653362.
381. Le Chevalier T, Ibrahim N, Chomy P, Riviere A, Mannier A, Magherini E, Pujol JL (1997) A phase II study of irinotecan in patients with small cell lung cancer progressing after first line treatment. Proc Amer Soc Clin Oncol 16: 450a
382. Devore R, Blanke C, Denham C, Hainsworth JD, Gralla RJ, Koletsky AJ, Savaraj N, Vogal CL, Sarma GP, Brooks DJ, Petit RG, Elfing GL, Schaaf LJ, Hanover CK, Miller LL (1998) Phase II study of CPT-11 in patients with previously treated small cell lung cancer. Proc Amer Soc Clin Oncol 17: 451a
383. Kelly K. Irinotecan in small-cell lung cancer: current data. Clin Lung Cancer. 2001 May; 2 Suppl 2:S4-8. doi: 10.3816/clc.2001.s.001. PMID: 14725723.
384. Saijo, N. Progress in treatment of small-cell lung cancer: role of CPT-11. Br J Cancer 89, 2178-2183 (2003). https://doi.org/10.1038/sj.bjc.6601456
385. Vijay Maruti Patil, Vanita Noronha, Nandini Menon et.al. Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study. J Clin Oncol. 2023; 41(2): 222 - 232.
386. Kirill V Lepik, Liudmila V Fedorova, Elena V Kondakova et.al. A Phase 2 Study of Nivolumab Using a Fixed Dose of 40 mg (Nivo40) in Patients With Relapsed/Refractory Hodgkin Lymphoma. Hemasphere. 2020; 4(5):e480.
387. Suzanne L Topalian, F Stephen Hodi, Julie R Brahmer et.al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366(26):2443-54.
388. Shin Hye Yoo, Bhumsuk Keam, Miso Kim et.al. Low-dose nivolumab can be effective in non-small cell lung cancer: alternative option for financial toxicity. ESMO Open. 2018; 3(5):e000332.
389. Robert J Motzer, Brian I Rini, David F McDermott et.al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015; 33(13):1430-37.
390. Van den Heuvel, M., Van der Noort, V., Ter Heine, R., et al. (2024). Low dose versus standard dose pembrolizumab for treatment of stage IV non-small cell lung carcinoma: Results of the pre-planned interim analysis of the NVALT-30 clinical trial. Presented at: 2024 ESMO Congress; September 13 - 17, 2024; Barcelona, Spain. Abstract 1258MO
391. Mayer N, Boschetti L, Scarci M, Cioffi U, De Simone M, Schnider M, Kestenholz P, Minervini F. Brain Imaging in Patients with Non-Small Cell Lung Cancer-A Systematic Review. J Clin Med. 2025 Jan 22; 14(3):708. doi: 10.3390/jcm14030708. PMID: 39941379; PMCID: PMC11818832.
392. Tsao MN, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018; 1:CD003869.
393. Carmichael, J., Crane, J.M., Bunn, P.A., Glatstein, E. and Ihde, D.C. Results of therapeutic cranial irradiation in small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 14: 455 - 459, 1988.
394. Hagedorn, H.E., Haaxma-Reiche, H., Canrinus, A., Vermey, J., Smit, E.F. and Postmus, P.E. Results of whole brain radiotherapy for brain metastases of small cell lung cancer. Lung Cancer 8: 293 - 300, 1993.
395.  , Belda-Sanchis J, Guarino M, Tilea L, Cordero JVR, Mart nez-T llez E. The current role of surgery and SBRT in early stage of small cell lung cancer. J Clin Transl Res. 2021 Feb 17; 7(1): 34 - 48. PMID: 34104807; PMCID: PMC8177012.
, Belda-Sanchis J, Guarino M, Tilea L, Cordero JVR, Mart nez-T llez E. The current role of surgery and SBRT in early stage of small cell lung cancer. J Clin Transl Res. 2021 Feb 17; 7(1): 34 - 48. PMID: 34104807; PMCID: PMC8177012.
396. Rathod S, Koul R, Bashir B, Chowdhury A, Dubey A. Role of Stereotactic Body Radiation Therapy in Early Stage Small Cell Lung Cancer in the Era of Lung Cancer Screening: A Systematic Review. Am J Clin Oncol. 2019; 42(2): 123 - 130. doi:10.1097/COC.0000000000000489
397. J Klastersky, J P Sculier, H Lacroix, G Dabouis, G Bureau, P Libert, M Richez, P Ravez, G Vandermoten, J Thiriaux, et al. A randomized study comparing cisplatin or carboplatin with etoposide in patients with advanced non-small-cell lung cancer: European Organization for Research and Treatment of Cancer Protocol 07861. PMID: 2167953 DOI: 10.1200/JCO.1990.8.9.1556
398. Sachiko Ozone, Kazuya Ichikawa, Masahiro Morise, Akira Matsui, Fumie Kinoshita, Reiko Matsuzawa, Junji Koyama, Ichidai Tanaka, Naozumi Hashimoto. Is area under the curve the best parameter for carboplatin induced emetic risk stratification? PMID: 34916721 PMCID: PMC8648517 DOI: 10.18999/nagjms.83.4.773
399. Sederholm C, Hillerdal G, Lamberg K, K lbeck K, Dufmats M, Westberg R, Gawande SR. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol. 2005 Nov 20; 23(33):8380-8. doi: 10.1200/JCO.2005.01.2781. PMID: 16293868.
400. Martin Reck, Tony S K Mok, Makoto Nishio et.al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations orbaseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019 May; 7(5): 387 - 401.
- Гражданский кодекс (ГК РФ)
- Жилищный кодекс (ЖК РФ)
- Налоговый кодекс (НК РФ)
- Трудовой кодекс (ТК РФ)
- Уголовный кодекс (УК РФ)
- Бюджетный кодекс (БК РФ)
- Арбитражный процессуальный кодекс
- Конституция РФ
- Земельный кодекс (ЗК РФ)
- Лесной кодекс (ЛК РФ)
- Семейный кодекс (СК РФ)
- Уголовно-исполнительный кодекс
- Уголовно-процессуальный кодекс
- Производственный календарь на 2025 год
- МРОТ 2026
- ФЗ «О банкротстве»
- О защите прав потребителей (ЗОЗПП)
- Об исполнительном производстве
- О персональных данных
- О налогах на имущество физических лиц
- О средствах массовой информации
- Производственный календарь на 2026 год
- Федеральный закон "О полиции" N 3-ФЗ
- Расходы организации ПБУ 10/99
- Минимальный размер оплаты труда (МРОТ)
- Календарь бухгалтера на 2026 год
- Частичная мобилизация: обзор новостей
- Постановление Правительства РФ N 1875