1. Neufeld E., Muenzer J. The mucopolysaccharidoses//In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, eds. The Metabolic and Molecular Basis of Inherited Disease. 8 ed. New York. NY: McGraw-Hill. 2001. P. 3421 - 52.
2. Scarpa M., Almassy Z., Beck M. et al. Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease//Orphanet J Rare Dis. 2011. V. 6. P. 72 - 7.
3. Muenzer J., Jones S.A., Tylki-Szymanska A. et al. Ten years of the Hunter Outcome Survey (HOS): insights, achievements, and lessons learned from a global patient registry//Orphanet J Rare Dis. 2017. V. 12. N 1. P. 82.
4. Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders//JAMA. 1999. V. 281. P. 249 - 54.
5. Martin R., Beck M., Eng C., Giugliani R., Harmatz P., Mufioz V. et al. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome)//Pediatrics. 2008. V. 121. P. 377 - 86.
6. Simmons M.A., Bruce I.A., Penney S., Wraith E., Rothera M.P. Otorhinolaryngological manifestations of the mucopolysaccharidoses//Int. J. Pediatr. Otorhinolaryngol. 2005. V. 69. P. 589 - 95.
7. Biswas J., Nandi K., Sridharan S., Ranjan P. Ocular manifestation of storage diseases//Curr. Opin. Ophthalmol. 2008. V. 19. P. 507 - 11.
8. Thappa D. M., Singh A., Jaisankar T. J., Rao R., Ratnakar C. Pebbling of the Skin: A Marker of Hunter's Syndrome//Pediatric Dermatology. 1998. V. 15. N 5. P. 370 - 3.
9. Barbier A.J., Bielefeld B., Whiteman D.A. et al. The relationship between anti-idursulfase antibody status and safety and efficacy outcomes in attenuated mucopolysaccharidosis II patients aged 5 years and older treated with intravenous idursulfase//Mol Genet Metab. 2013. V. 110. N 3. P. 303 - 10.
10. Kim C., Seo J., Chung Y., Ji H.J., Lee J., Sohn J. Comparative study of idursulfase beta and idursulfase in vitro and in vivo//J. Hum. Genet. 2017. V. 62. P. 167 - 74.
11. Miebach E. Management of infusion-related reactions to enzyme replacement therapy in a cohort of patients with mucopolysaccharidosis disorders//Int J Clin Pharmacol Ther. 2009. V. 47. N 1. P. 100 - 6.
12. Wraith J.E., Scarpa M., Beck M. et al. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy//Eur J Pediatr. 2008. V. 167. P. 267 - 77.
13. Sampayo-Cordero M., Miguel-Huguet B., Pardo-Mateos A. Agreement between results of meta-analyses from case reports and clinical studies, regarding efficacy and safety of idursulfase therapy in patients with mucopolysaccharidosis type II (MPS-II). A new tool for evidence-based medicine in rare diseases//Orphanet J Rare Dis. 2019. V. 14. N 1. P. 230.
14. Bradley L., Haddow H., Palomaki G. Treatment of mucopolysaccharidosis type II (Hunter syndrome): results from a systematic evidence review//Genet Med. 2017. V. 19. P. 1187 - 1201.
15. Da Silva E. M. K. et al. Enzyme replacement therapy with idursulfase for mucopolysaccharidosis type II (Hunter syndrome)//Cochrane Database of Systematic Reviews. 2016. N 2.
16. Giugliani R., Federhen A., Rojas M.V. et al. Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment//Genet Mol Biol. 2010. V. 33. N 4. P. 589 - 604.
17. Rezende M. M. et al. Brazilian reference values for MPS II screening in dried blood spots-A fluorimetric assay//Clinical biochemistry. 2014. V. 47. N 13 - 14. P. 1297 - 9.
18. Human Gene Mutations Database. Qiagen HGMD Professional.
19. Okuyama T., Tanaka A., Suzuki Y. et al. Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (mucopolysaccharidosis II, MPS II)//Mol Genet Metab. 2010. V. 99. P. 18 - 25.
20. Guffon N., Heron B., Chabrol B. Diagnosis, quality of life, and treatment of patients with Hunter syndrome in the French healthcare system: a retrospective observational study//Orphanet J Rare Dis. 2015. V. 10. P. 43.
21. Jurecka A., Zuberuber Z., Opoka-Winiarska V. et al. Effect of rapid cessation of enzyme replacement therapy: a report of 5 cases and a review of the literature//Mol Genet Metab. 2012. V. 107. P. 508 - 12.
22. Миронов С.П., Колесов С.В., Переверзев В.С., Колбовский Д.А., Кулешов А.А., Ветрилэ М.С., Казьмин А.И. Опыт хирургического лечения краниовертебрального стеноза у пациентов с мукополисахаридозом I, II, VI типов//Хирургия позвоночника. 2018. Т. 15. N 4. С. 32 - 40.
23. Williams N., Challoumas D., Eastwood D. M. Does orthopaedic surgery improve quality of life and function in patients with mucopolysaccharidoses?//Journal of Children's Orthopaedics. 2017. V. 11. N 4. P. 289 - 97.
24. Miebach E. Management of infusion-related reactions to enzyme replacement therapy in a cohort of patients with mucopolysaccharidosis disorders//International journal of clinical pharmacology and therapeutics. 2009. V. 47. P. S100 - 6.
25. Mendelsohn N. J., Harmatz P., Bodamer O. et al. Importance of surgical history in diagnosing mucopolysaccharidosis type II (Hunter syndrome): Data from the Hunter Outcome Survey//Genetics in Medicine. 2010. V. 12. N 12. P. 816 - 22.
26. Remondino R. G. et al. Clinical Manifestations and Surgical Management of Spinal Lesions in Patients With Mucopolysaccharidosis: A Report of 52 Cases//Spine deformity. 2019. V. 7. N. 2. P. 298 - 303.
27. Kwon JY, Ko K, Sohn YB, et al. High prevalence of carpal tunnel syndrome in children with mucopolysaccharidosis type II (Hunter syndrome). Am J Med Genet A. 2011; 155A: 1329 - 1335.
28. ATS statement: Guidelines for the Six-Minute Walk Test. American Journal of Respiratory and Critical Care Medicine. Vol. 166, No. 1. Jul 01, 2002.
29. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, Khan DA, Lang DM, Park HS, Pichler W, Sanchez-Borges M, Shiohara T, Thong BY. International Consensus on drug allergy. Allergy. 2014 Apr; 69(4): 420 - 37.
30. Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, Lockey RF, El-Gamal YM, Brown SG, Park HS, Sheikh A. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015 Oct 28; 8(1): 32.
31. Ильина Н.И., и соавт. Анафилактический шок (2-й пересмотр). Клинические рекомендации Российской ассоциации аллергологов и клинических иммунологов и Общероссийской общественной организации "Федерация анестезиологов и реаниматологов"//Вестник интенсивной терапии имени А.И. Салтанова. 2024.
32. Bitencourt FH, Vieira TA, Steiner CE, Neto JC, Boy R, Schwartz IVD. Medical Costs Related to Enzyme Replacement Therapy for Mucopolysaccharidosis Types I, II, and VI in Brazil: A Multicenter Study. Value Health Reg Issues. 2015 Dec; 8: 99 - 106.
33. https://emedicine.medscape.com/article/944723-followup
34. Вашакмадзе Н.Д. Мультидисциплинарные принципы ведения детей с мукополисахаридозами в повышении эффективности их диагностики и лечения: автореферат диссертации доктора медицинских наук: 14.01.08, Екатеринбург, 2019. - 47 с.
35. Franco JFDS, El Dib R, Agarwal A, Soares D, Milhan NVM, Albano LMJ, Kim CA. Mucopolysaccharidosis type I, II and VI and response to enzyme replacement therapy: Results from a single-center case series study. Intractable Rare Dis Res. 2017 Aug; 6(3): 183 - 190.
36. Keilmann A, Bendel F, Nospes S, Lampe C,  AK. Alterations of mucosa of the larynx and hypopharynx in patients with mucopolysaccharidoses. J Laryngol Otol. 2016 Feb; 130(2): 194 - 200.
AK. Alterations of mucosa of the larynx and hypopharynx in patients with mucopolysaccharidoses. J Laryngol Otol. 2016 Feb; 130(2): 194 - 200.
37. Berger KI, Fagondes SC, Giugliani R, Hardy KA, Lee KS, McArdle C, Scarpa M, Tobin MJ, Ward SA, Rapoport DM. Respiratory and sleep disorders in mucopolysaccharidosis. J Inherit Metab Dis. 2013 Mar; 36(2): 201 - 10.
38. Scarpa M, 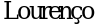 CM, Amartino H. Epilepsy in mucopolysaccharidosis disorders. Mol Genet Metab. 2017 Dec; 122S: 55 - 61.
CM, Amartino H. Epilepsy in mucopolysaccharidosis disorders. Mol Genet Metab. 2017 Dec; 122S: 55 - 61.
39. Taccone A, Tortori Donati P, Marzoli A, Dell'Acqua A, Gatti R, Leone D. Mucopolysaccharidosis: thickening of dura mater at the craniocervical junction and other CT/MRI findings. Pediatr Radiol. 1993; 23(5): 349 - 52, Sheridan M, Johnston I. Hydrocephalus and pseudotumour cerebri in the mucopolysaccharidoses. Childs Nerv Syst. 1994 Apr; 10(3): 148 - 50.
40. Lin HY, Shih SC, Chuang CK, Chen MR, Niu DM, Lin SP. Assessment of bone mineral density by dual energy x-ray absorptiometry in patients with mucopolysaccharidoses. Orphanet J Rare Dis. 2013 May 11; 8: 71.
41. Jiang Z, Byers S, Casal ML, Smith LJ. Failures of Endochondral Ossification in the Mucopolysaccharidoses. Curr Osteoporos Rep. 2020 Dec; 18(6): 759 - 773.
42. Burton BK, Whiteman DA; HOS Investigators. Incidence and timing of infusion-related reactions in patients with mucopolysaccharidosis type II (Hunter syndrome) on idursulfase therapy in the real-world setting: a perspective from the Hunter Outcome Survey (HOS). Mol Genet Metab. 2011 Jun; 103(2): 113 - 20.
43. Escolar ML, Jones SA, Shapiro EG, Horovitz DDG, Lampe C, Amartino H. Practical management of behavioral problems in mucopolysaccharidoses disorders. Mol Genet Metab. 2017 Dec; 122S: 35 - 40.
44. Giugliani R, Villarreal ML, Valdez CA, Hawilou AM, Guelbert N,  LN, Martins AM, Acosta A, Cabello JF, Lemes A, Santos ML, Amartino H. Guidelines for diagnosis and treatment of Hunter Syndrome for clinicians in Latin America. Genet Mol Biol. 2014 Jun; 37(2): 315 - 29.
LN, Martins AM, Acosta A, Cabello JF, Lemes A, Santos ML, Amartino H. Guidelines for diagnosis and treatment of Hunter Syndrome for clinicians in Latin America. Genet Mol Biol. 2014 Jun; 37(2): 315 - 29.
45. Congedi S, Orzalesi M, Di Pede C, Benini F. Pain in Mucopolysaccharidoses: Analysis of the Problem and Possible Treatments. Int J Mol Sci. 2018 Oct 8; 19(10): 3063.
46. Venekamp RP, Hearne BJ, Chandrasekharan D, Blackshaw H, Lim J, Schilder AG. Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children. Cochrane Database Syst Rev. 2015 Oct 14; (10): CD011165.
47. Bianchi PM, Gaini R, Vitale S. ENT and mucopolysaccharidoses. Ital J Pediatr. 2018 Nov 16; 44(Suppl 2): 127.
48. Yang L, Shan Y, Wang S, Cai C, Zhang H. Endoscopic assisted adenoidectomy versus conventional curettage adenoidectomy: a meta-analysis of randomized controlled trials. Springerplus. 2016 Apr 11; 5: 426.
49. Harrison R, Schaefer S, Warner L, Mercer J, Jones S, Bruce I. Transnasal adenoidectomy in mucopolysaccharidosis. Int J Pediatr Otorhinolaryngol. 2018 Aug; 111: 149 - 152.
50. Mitchell RB, Archer SM, Ishman SL, Rosenfeld RM, Coles S, Finestone SA, Friedman NR, Giordano T, Hildrew DM, Kim TW, Lloyd RM, Parikh SR, Shulman ST, Walner DL, Walsh SA, Nnacheta LC. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol Head Neck Surg. 2019 Feb; 160(1_suppl): S1 - S42.
51. Murgasova L, Jurovcik M, Jesina P, Malinova V, Bloomfield M, Zeman J, Magner M. Otorhinolaryngological manifestations in 61 patients with mucopolysaccharidosis. Int J Pediatr Otorhinolaryngol. 2020 Aug; 135: 110137.
52. Носко А.С., Зыков В.П., Новикова Е.Б. Алгоритм дифференциальной диагностики неврологических нарушений у пациентов с синдромом Хантера//Поликлиника. - 2022. - N 1. - С. 20 - 23.
53. Holt J., Poe M.D., Escolar M.L. Early Clinical Markers of Central Nervous System Involvement in Mucopolysaccharidosis Type II//J Pediatr. 2011. Vol. 159, N 2. P. 320 - 326. e2.
54. Roid G.H., Koch C. Leiter-3: Nonverbal Cognitive and Neuropsychological Assessment//Handbook of Nonverbal Assessment. Cham: Springer International Publishing, 2017. P. 127 - 150.
55. Alcantud F., Alonso Y. (2016). Predictive value of the Merrill-Palmer-R Scale applied during the first year of live [sic]. Psicologia Educativa, 22, 87 - 92. https://doi.org/10.1016/j.pse.2016.01.001 https://journals.copmadrid.org/psed/art/j.pse.2016.01.001.
56. Syeda M. M., Climie E. A. Test Review: Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition. - 2014.
57. Тест Векслера. Диагностика уровня развития интеллекта (детский вариант): методическое руководство/Ю.И. Филимоненко, В.И. Тимофеев. - СПб: ИМАТОНб 2012. - 112 с. ISBN 5-7822-0037-5.
58. Lichtenberger E.O., Kaufman A.S. Kaufman Assessment Battery for Children-Second Edition (KABC-II)//Encyclopedia of Cross-Cultural School Psychology. Boston, MA: Springer US, 2010. P. 557 - 560.
59. Sparrow S. S., Cicchetti D. V., Saulnier C. A. Vineland adaptive behavior scales, (Vineland-3)//Antonio: Psychological Corporation. - 2016.
60. Овчинникова Ирина Викторовна. Апробация методики Vineland Adaptive Behavior Scales (VABS) на русскоязычной выборке/Вопросы психологии. - 2018. - N 6. - С. 134 - 145.
61. Mullen E. M. et al. Mullen scales of early learning. - Circle Pines, MN: AGS, 1995. - С. 58 - 64.
62. Seo J.-H. et al. Intracerebroventricular enzyme replacement therapy in patients with neuronopathic mucopolysaccharidosis type II: Final report of 5-year results from a Japanese open-label phase 1/2 study//Mol Genet Metab. 2023. Vol. 140, N 4. P. 107709.
63. Инструкция по медицинскому применению препарата Хантераза ЛП N (002923)-(РГ-RU).
64. Sohn Y.B. et al. Safety and efficacy of enzyme replacement therapy with idursulfase beta in children aged younger than 6years with Hunter syndrome//Mol Genet Metab. 2015. Vol. 114, N 2. P. 156 - 160.
65. Yoon Ji Ahn et al., PO-219 two-year safety and efficacy extension study of GC1111 in MPS-II patients treated with GC1111 or idursulfase during phase 3 study, Journal Of Inherited Metabolic Disease, Volume 47, No. S1, August 2024, p. 194.
66. Jeffrey D. Kingsley et al. Immunizations for Patients With Metabolic Disorders. Pediatrics (2006) 118 (2): e460 - e470. https://doi.org/10.1542/peds.2005-1257
67. Fr. Menni, G. Chiarelli, C. Sabatini, N. Principi, S. Esposito. Vaccination in children with inborn errors of metabolism. Vaccine. 2012; 30 (50): 7161 - 7164.
68. N.P. Klein et al. Evaluation of Immunization Rates and Safety Among Children With Inborn Errors of Metabolism. Pediatrics (2011) 127 (5): e1139 - e1146. https://doi.org/10.1542/peds.2010-3706
69. Ramos BCF, Aranda CS, Cardona RSB, Martins AM, ![]() D, Clemens SAC, Clemens R. Vaccination strategies for people living with inborn errors of metabolism in Brazil. J Pediatr (Rio J). 2023 Mar - Apr; 99 Suppl 1 (Suppl 1): S70 - S80. doi: 10.1016/j.jped.2022.12.001
D, Clemens SAC, Clemens R. Vaccination strategies for people living with inborn errors of metabolism in Brazil. J Pediatr (Rio J). 2023 Mar - Apr; 99 Suppl 1 (Suppl 1): S70 - S80. doi: 10.1016/j.jped.2022.12.001
70. Приказ Минздрава России от 06.12.2021 N 1122н "Об утверждении национального календаря профилактических прививок, календаря профилактических прививок по эпидемическим показаниям и порядка проведения профилактических прививок".
71. Методические указания МУ 3.3.1.1095-02. Медицинские противопоказания к проведению профилактических прививок препаратами национального календаря прививок.
72. Федосеенко М.В., Намазова-Баранова Л.С., Вишнева Е.А., Толстова С.В., Сельвян А.М., Калюжная Т.А., Шахтахтинская Ф.Ч., Солошенко М.А., Привалова Т.Е., Фоминых М.В., Зиновьева Т.Е. Совершенствование подходов к иммунопрофилактике детей с отклонениями в состоянии здоровья: результаты проспективного когортного исследования. Педиатрическая фармакология. 2021; 18(6): 470 - 483. doi: 10.15690/pf.v18i6.2328
73. Baumer T, Buhring N, Schelle T, Munchau A, Muschol N. Nerve ultrasound in clinical management of carpal tunnel syndrome in mucopolysaccharidosis. Dev Med Child Neurol. 2016 doi: 10.1111/dmcn.13127
74. Jester AAM. Ultrasonography for diagnosis and follow-up of carpal tunnel syndrome in mucopolysaccharidosis. Dev Med Child Neurol. 2016 doi: 10.1111/dmcn.13148
75. Patel P, Antoniou G, Clark D, Ketteridge D, Williams N. Screening for Carpal Tunnel Syndrome in Patients With Mucopolysaccharidosis. J Child Neurol. 2020 May; 35(6): 410 - 417. doi: 10.1177/0883073820904481. Epub 2020 Mar 11. PMID: 32157938; PMCID: PMC7153223
76. Zairi M, Msakni A, Mohseni AA, Nessib N, Bouali S, Boussetta R, Nessib MN. Cranio-cervical decompression associated with non-instrumented occipito-C2 fusion in children with mucopolysaccharidoses: Report of twenty-one cases. N Am Spine Soc J. 2022 Nov 19; 12: 100183. doi: 10.1016/j.xnsj.2022.100183. PMID: 36458130; PMCID: PMC9706171
77. Liu HT, Song J, Zhou FC, Liang ZH, Zhang QQ, Zhang YH, Shao J. Cervical spine involvement in pediatric mucopolysaccharidosis patients: Clinical features, early diagnosis, and surgical management. Front Surg. 2023 Jan 6; 9: 1059567. doi: 10.3389/fsurg.2022.1059567. PMID: 36684186; PMCID: PMC9852728
78. Mao, SJ., Chen, QQ., Dai, YL. et al. The diagnosis and management of mucopolysaccharidosis type II. Ital J Pediatr 50, 207 (2024). https://doi.org/10.1186/s13052-024-01769-9
79. Sohn YB, Cho SY, Park SW, Kim SJ, Ko AR, Kwon EK, Han SJ, Jin DK. Phase I/II clinical trial of enzyme replacement therapy with idursulfase beta in patients with mucopolysaccharidosis II (Hunter syndrome). Orphanet J Rare Dis. 2013 Mar 18; 8: 42. doi: 10.1186/1750-1172-8-42. PMID: 23497636; PMCID: PMC3614543
80. Sohn YB, Cho SY, Lee J, Kwun Y, Huh R, Jin DK. Safety and efficacy of enzyme replacement therapy with idursulfase beta in children aged younger than 6 years with Hunter syndrome. Mol Genet Metab. 2015 Feb; 114(2): 156 - 60. doi: 10.1016/j.ymgme.2014.08.009. Epub 2014 Aug 30. PMID: 25219292
81. Seo JH, Kosuga M, Hamazaki T, Shintaku H, Okuyama T. Impact of intracerebroventricular enzyme replacement therapy in patients with neuronopathic mucopolysaccharidosis type II. Mol Ther Methods Clin Dev. 2021 Feb 27; 21: 67 - 75. doi: 10.1016/j.omtm.2021.02.018. PMID: 33768130; PMCID: PMC7957024
82. Gnasso R, Corrado B, Iommazzo I, Migliore F, Magliulo G, Giardulli B, Ruosi C. Assessment, pharmacological therapy and rehabilitation management of musculoskeletal pain in children with mucopolysaccharidoses: a scoping review. Orphanet J Rare Dis. 2022 Jul 8; 17(1): 255. doi: 10.1186/s13023-022-02402-w. PMID: 35804400; PMCID: PMC9264657
83. Harshanand P1, Anil Kumar G, Vivek P, Jayasree R Mucopolysaccharidosis and Rehabilitation October 2013Indian Journal of Physical Medicine and Rehabilitation 24 (20(june 2013): 44.
84.  Z,
Z,  B,
B, 
![]() , Wierzba J,
, Wierzba J,  A. Diagnosis and Management of Mucopolysaccharidosis Type II (Hunter Syndrome) in Poland. Biomedicines. 2023 Jun 8; 11(6): 1668. doi: 10.3390/biomedicines11061668. PMID: 37371763; PMCID: PMC10296388
A. Diagnosis and Management of Mucopolysaccharidosis Type II (Hunter Syndrome) in Poland. Biomedicines. 2023 Jun 8; 11(6): 1668. doi: 10.3390/biomedicines11061668. PMID: 37371763; PMCID: PMC10296388
85. Muenzer J, Beck M, Eng CM, Escolar ML, Giugliani R, Guffon NH, Harmatz P, Kamin W, Kampmann C, Koseoglu ST, Link B, Martin RA, Molter DW,  Rojas MV, Ogilvie JW, Parini R, Ramaswami U, Scarpa M, Schwartz IV, Wood RE, Wraith E. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009 Dec; 124(6): e1228 - 39. doi: 10.1542/peds.2008-0999. Epub 2009 Nov 9. PMID: 19901005
Rojas MV, Ogilvie JW, Parini R, Ramaswami U, Scarpa M, Schwartz IV, Wood RE, Wraith E. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009 Dec; 124(6): e1228 - 39. doi: 10.1542/peds.2008-0999. Epub 2009 Nov 9. PMID: 19901005
86. Stapleton M, Kubaski F, Mason RW, Yabe H, Suzuki Y, Orii KE, Orii T, Tomatsu S. Presentation and Treatments for Mucopolysaccharidosis Type II (MPS II; Hunter Syndrome). Expert Opin Orphan Drugs. 2017; 5(4): 295 - 307. doi: 10.1080/21678707.2017.1296761. Epub 2017 Mar 8. PMID: 29158997; PMCID: PMC5693349
87. Oussoren E, Mathijssen IMJ, Wagenmakers M, Verdijk RM, Bredero-Boelhouwer HH, van Veelen-Vincent MC, van der Meijden JC, van den Hout JMP, Ruijter GJG, van der Ploeg AT, Langeveld M. Craniosynostosis affects the majority of mucopolysaccharidosis patients and can contribute to increased intracranial pressure. J Inherit Metab Dis. 2018 Nov; 41(6): 1247 - 1258. doi: 10.1007/s10545-018-0212-1. Epub 2018 Aug 6. PMID: 30083803; PMCID: PMC6326980
88. Резолюция по итогам экспертного совета Педиатрическая фармакология/2024/том 21/N 1 с. 66 - 69 https://doi.org/10.15690/pf.v21i1.2657
89. https://www-uptodate-com.libook.xyz/contents/new-onset-urticaria-hives?search=cetirizine%20pediatric%20dosing&source=search_result&selectedTitle=5~98&usage_type=default&display_rank=4
- Гражданский кодекс (ГК РФ)
- Жилищный кодекс (ЖК РФ)
- Налоговый кодекс (НК РФ)
- Трудовой кодекс (ТК РФ)
- Уголовный кодекс (УК РФ)
- Бюджетный кодекс (БК РФ)
- Арбитражный процессуальный кодекс
- Конституция РФ
- Земельный кодекс (ЗК РФ)
- Лесной кодекс (ЛК РФ)
- Семейный кодекс (СК РФ)
- Уголовно-исполнительный кодекс
- Уголовно-процессуальный кодекс
- Производственный календарь на 2025 год
- МРОТ 2025
- ФЗ «О банкротстве»
- О защите прав потребителей (ЗОЗПП)
- Об исполнительном производстве
- О персональных данных
- О налогах на имущество физических лиц
- О средствах массовой информации
- Производственный календарь на 2026 год
- Федеральный закон "О полиции" N 3-ФЗ
- Расходы организации ПБУ 10/99
- Минимальный размер оплаты труда (МРОТ)
- Календарь бухгалтера на 2025 год
- Частичная мобилизация: обзор новостей
- Постановление Правительства РФ N 1875