1. Packham G., Stevenson F. The role of the B-cell receptor in the pathogenesis of chronic lymphocytic leukaemia//Seminars in Cancer Biology. 2010. Vol. 20, N 6. P. 391 - 399.
2. Vardi A. et al. Immunogenetic studies of chronic lymphocytic leukemia: Revelations and speculations about ontogeny and clinical evolution//Cancer Research. American Association for Cancer Research Inc., 2014. Vol. 74, N 16. P. 4211 - 4216.
3. Murray F. et al. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: Implications for the role of antigen selection in leukemogenesis//Blood. 2008. Vol. 111, N 3. P. 1524 - 1533.
4. Strati P., Shanafelt T.D. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: Diagnosis, natural history, and risk stratification//Blood. American Society of Hematology, 2015. Vol. 126, N 4. P. 454 - 462.
5. Zhang S., Kipps T.J. The Pathogenesis of Chronic Lymphocytic Leukemia//Annual Review of Pathology: Mechanisms of Disease. 2014. Vol. 9, N 1. P. 103 - 118.
6. Burger J.A., Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia//Trends in Immunology. 2013. Vol. 34, N 12. P. 592 - 601.
7. Morton L.M. et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992 - 2001//Blood. 2006. Vol. 107, N 1. P. 265 - 276.
8. Watson L., Wyld P., Catovsky D. Disease burden of chronic lymphocytic leukaemia within the European Union//Eur J Haematol. 2008. Vol. 81, N 4. P. 253 - 258.
9. Jemal A. et al. Cancer statistics, 2007.//CA Cancer J Clin. 2007. Vol. 57, N 1. P. 43 - 66.
10. Dores G.M. et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: Overview of the descriptive epidemiology//Br J Haematol. 2007. Vol. 139, N 5. P. 809 - 819.
11. Злокачественные новообразования в России в 2017 году (заболеваемость и смертность). Под ред. А.Д. Каприна, В.В. Старинского, Г.В. Петровой. М.: МНИОИ им. П.А. Герцена - филиал ФГБУ "НМИЦ радиологии" Минздрава России, 2018.
12. Hallek M. et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL//Blood. American Society of Hematology, 2018. Vol. 131, N 25. P. 2745 - 2760.
13. Armitage J.O. Staging Non-Hodgkin Lymphoma//CA Cancer J Clin. Wiley, 2005. Vol. 55, N 6. P. 368 - 376.
14. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data//Lancet Oncol. Lancet Publishing Group, 2016. Vol. 17, N 6. P. 779 - 790.
15. Swerdlow S.H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms//Blood. 2016.
16. Никитин Е.А. et al. Хронический лимфолейкоз.//Российские клинические рекомендации по диагностике и лечению злокачественных лимфопролиферативных заболеваний; под ред. И.В. Поддубной, В.Г. Савченко. 2018. P. 179 - 200.
17. Мещерякова Л.Н. et al. Лабораторные возможности дифференциальной диагностики анемий//Онкогематология. 2015. Vol. 10, N 2. P. 46 - 50.
18. Morice W.G. et al. Predictive value of blood and bone marrow flow cytometry in B-cell lymphoma classification: Comparative analysis of flow cytometry and tissue biopsy in 252 patients//Mayo Clin Proc. Elsevier Ltd, 2008. Vol. 83, N 7. P. 776 - 785.
19. Rawstron A.C. et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia//New England Journal of Medicine. Massachussetts Medical Society, 2008. Vol. 359, N 6. P. 575 - 583.
20. Molica S., Giannarelli D., Montserrat E. Minimal Residual Disease and Survival Outcomes in Patients With Chronic Lymphocytic Leukemia: A Systematic Review and Meta-analysis.//Clin Lymphoma Myeloma Leuk. 2019. Vol. 19, N 7. P. 423 - 430.
21. Sharman J.P. et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial//The Lancet. Lancet Publishing Group, 2020. Vol. 395, N 10232. P. 1278 - 1291.
22. Fischer K. et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions//N Engl J Med. Massachussetts Medical Society, 2019. Vol. 380, N 23. P. 2225 - 2236.
23. Wierda W.G. et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study//J Clin Oncol. J Clin Oncol, 2021. Vol. 39, N 34. P. 3853 - 3865.
24. Jain N. et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL//N Engl J Med. N Engl J Med, 2019. Vol. 380, N 22. P. 2095 - 2103.
25. Rossi D. et al. Occult hepatitis B virus infection of peripheral blood mononuclear cells among treatment-na ve patients with chronic lymphocytic leukemia//Leuk Lymphoma. 2009. Vol. 50, N 4. P. 604 - 611.
26. Conte M.J. et al. Use of positron emission tomography-computed tomography in the management of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma//Leuk Lymphoma. Informa Healthcare, 2014. Vol. 55, N 9. P. 2079 - 2084.
27. Parikh S.A., Kay N.E., Shanafelt T.D. How we treat Richter syndrome.//Blood. 2014. Vol. 123, N 11. P. 1647 - 1657.
28. Bodey G.P., Kontoyiannis D., Keating M.J. Infections in Patients With Chronic Lymphocytic Leukemia//Chronic Lymphocytic Leukemia. Totowa, NJ: Humana Press, 2004. P. 343 - 359.
29. Клясова Г.А. et al. Возбудители сепсиса у иммунокомпрометированных больных: структура и проблемы антибиотикорезистентности (результаты многоцентрового исследования)//Гематология и трансфузиология. 2007. Vol. 52, N 1. P. 11 - 18.
30. Tam C.S. et al. SEQUOIA: Results of a Phase 3 Randomized Study of Zanubrutinib versus Bendamustine + Rituximab (BR) in Patients with Treatment-Na ve (TN) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL)//Blood. 2021. Vol. 138, N Supplement 1. P. 396 - 396.
31. Tam C.S. et al. Zanubrutinib monotherapy for patients with treatment na ve chronic lymphocytic leukemia and 17p deletion//Haematologica. Haematologica, 2020. Vol. 106, N 9. P. 2354 - 2363.
32. Shanafelt T., Wang V., Kay N. A randomized phase III study of ibrutinib (PCI-32765)-based therapy vs. standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy in untreated younger patients with chronic lymphocytic leukemia (CLL): a trial of the ECOG-ACRIN Cancer//ASH Annual Meeting. 2018. P. LBA-4.
33. Burger J.A. et al. Ibrutinib for First-Line Treatment of Older Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): A 4-Year Experience From the REASONATE-2 Study//EHA 23 Proceedings. 2018. P. Abstract PF343.
34. Burger J.A. et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia.//Blood. 2019. Vol. 133, N 10. P. 1011 - 1019.
35. Woyach J.A. et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL.//N Engl J Med. 2018. Vol. 379, N 26. P. 2517 - 2528.
36. Woyach J.A. et al. Acalabrutinib plus Obinutuzumab in 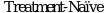 and Relapsed/Refractory Chronic Lymphocytic Leukemia//Cancer Discov. Cancer Discov, 2020. Vol. 10, N 3. P. 394 - 405.
and Relapsed/Refractory Chronic Lymphocytic Leukemia//Cancer Discov. Cancer Discov, 2020. Vol. 10, N 3. P. 394 - 405.
37. Davids M.S. et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study//Lancet Oncol. Lancet Oncol, 2021. Vol. 22, N 10. P. 1391 - 1402.
38. Eichhorst B. et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial//Lancet Oncol. Lancet Publishing Group, 2016. Vol. 17, N 7. P. 928 - 942.
39. Goede V. et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study//Leukemia. Leukemia, 2015. Vol. 29, N 7. P. 1602 - 1604.
40. Moreno C. et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial//Lancet Oncol. 2019. Vol. 20, N 1. P. 43 - 56.
41. Sorror M.L. et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning//Journal of Clinical Oncology. 2008. Vol. 26, N 30. P. 4912 - 4920.
42. Dreger P. et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: Long-term clinical and MRD results of the German CLL Study Group CLL3X trial//Blood. 2010. Vol. 116, N 14. P. 2438 - 2447.
43. Инструкция по медицинскому применению лекарственного препарата Венклекста (ЛП-004678). 2021.
44. Никитин Е.А., Птушкин В.В. Иммунохимиотерапия в лечении хронического лимфолейкоза//Хронический лимфолейкоз. Современная диагностика и лечение. 2nd ed. / ed. Никитин Е.А., Птушкин В.В. Москва: ГЭОТАР-Медиа, 2023. P. 219 - 246.
45. Калашникова О.Б. et al. Современные возможности терапии хронического лимфолейкоза и клиническая практика//Хронический лимфолейкоз. Современная диагностика и лечение. 2nd ed. / ed. Никитин Е.А., Птушкин В.В. Москва: ГЭОТАР-Медиа, 2023. P. 398 - 430.
46. Hallek M. et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial//The Lancet. Lancet Publishing Group, 2010. Vol. 376, N 9747. P. 1164 - 1174.
47. Fischer K. et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial//Blood. American Society of Hematology, 2016. Vol. 127, N 2. P. 208 - 215.
48. Assouline S. et al. Pharmacokinetics, safety, and efficacy of subcutaneous versus intravenous rituximab plus chemotherapy as treatment for chronic lymphocytic leukaemia (SAWYER): a phase 1b, open-label, randomised controlled non-inferiority trial.//Lancet Haematol. 2016. Vol. 3, N 3. P. e128 - 38.
49. Fischer K. et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: A multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group//Journal of Clinical Oncology. 2012. Vol. 30, N 26. P. 3209 - 3216.
50. Michallet A.S. et al. Rituximab plus bendamustine or chlorambucil for chronic lymphocytic leukemia: Primary analysis of the randomized, open-label mable study//Haematologica. Ferrata Storti Foundation, 2018. Vol. 103, N 4. P. 698 - 706.
51. Nikitin E. Randomised Comparison Of FCR-Lite And ClbR (Chlorambucil Plus Rituximab) Regimens In Elderly Patients With Chronic Lymphocytic Leukemia//EHA 18 Proceedings. 2013. P. Abstract NS1147.
52. Xu W. et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study//Journal of hematology & oncology. - 2020. - Т. 13. - С. 1 - 12.
53. Hillmen P. et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study//Journal of Clinical Oncology. American Society of Clinical Oncology, 2014. Vol. 32, N 12. P. 1236 - 1241.
54. Goede V. et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions//New England Journal of Medicine. Massachussetts Medical Society, 2014. Vol. 370, N 12. P. 1101 - 1110.
55. Catovsky D., Else M., Richards S. Chlorambucil-still not bad: A reappraisal//Clinical Lymphoma, Myeloma and Leukemia. Elsevier Inc., 2011. Vol. 11, N SUPPL.1. P. S2.
56. Hainsworth J.D. et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: A phase II trial of the Minnie Pearl Cancer Research Network//Journal of Clinical Oncology. 2003. Vol. 21, N 9. P. 1746 - 1751.
57. Seymour J.F. et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia.//N Engl J Med. 2018. Vol. 378, N 12. P. 1107 - 1120.
58. Stilgenbauer S. et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study//Lancet Oncol. Lancet Publishing Group, 2016. Vol. 17, N 6. P. 768 - 778.
59. Ghia P. et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia//Journal of Clinical Oncology. American Society of Clinical Oncology (ASCO), 2020. Vol. 38, N 25. P. JCO.19.03355.
60. Hillmen P. et al. First Interim Analysis of ALPINE Study: Results of a Phase 3 Randomized Study of Zanubrutinib vs Ibrutinib in Patients with Relapsed/Refractory Chronic Lymphocytic Lymphoma//EHA Library. 2021. P. 330170.
61. Tam C.S. et al. Life After FCR: Outcomes of patients with chronic lymphocytic leukemia who progress after frontline treatment with Fludarabine, Cyclophosphamide and Rituximab.//Blood. 2014. P. 3059 - 3064.
62. Fischer K. et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: A Multicenter Phase II trial of the German Chronic Lymphocytic Leukemia Study Group//Journal of Clinical Oncology. 2011. Vol. 29, N 26. P. 3559 - 3566.
63. Chanan-Khan A. et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3 study//Lancet Oncol. Lancet Publishing Group, 2016. Vol. 17, N 2. P. 200 - 211.
64. Hallek M. et al. Three-year follow-up of patients with previously treated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) receiving ibrutinib plus bendamustine and rituximab (BR) versus placebo plus BR: an update of the HELIOS study.//Leuk Lymphoma. 2017. Vol. 58. P. 192 - 194.
65. Савченко В.Г. и др. Алгоритмы диагностики и протоколы лечения заболеваний системы крови. Москва: Практика, 2018. 1008 p.
66. Maertens J. et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients//Journal of Antimicrobial Chemotherapy. Oxford University Press, 2016. Vol. 71, N 9. P. 1 - 8.
67. Raanani P. et al. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis.//Leuk Lymphoma. 2009. Vol. 50, N 5. P. 764 - 772.
68. Константинова Т.С., Клясова Г.А., Капланов К.Д. Лечение и профилактика инфекционных осложнений у пациентов с лимфопролиферативными заболеваниями.//Российские клинические рекомендации по диагностике и лечению злокачественных лимфопролиферативных заболеваний; под ред. И.В. Поддубной, В.Г. Савченко. 2018. P. 289 - 311.
69. Rubin L.G. et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host.//Clin Infect Dis. 2014. Vol. 58, N 3. P. 309 - 318.
70. Sinisalo M. et al. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia.//Br J Haematol. 2001. Vol. 114, N 1. P. 107 - 110.
71. Pleyer C. et al. Response to the Shingrix Varicella Zoster Virus (VZV) Vaccine in Patients with Chronic Lymphocytic Leukemia (CLL) That Are Treatment Naive or Treated with a Bruton's Tyrosine Kinase Inhibitor (BTK-I)//Blood. Content Repository Only!, 2019. Vol. 134, N Supplement_1. P. 3053.
72. Busti F. et al. Anemia and iron deficiency in cancer patients: Role of iron replacement therapy//Pharmaceuticals. MDPI AG, 2018. Vol. 11, N 4.
73. Rai K.R., Stilgenbauer S. Overview of the complications of chronic lymphocytic leukemia [Electronic resource]. URL: https://www.uptodate.com/contents/overview-of-the-complications-of-chronic-lymphocytic-leukemia (accessed: 30.10.2020).
74. Paul K.L. Rehabilitation and exercise considerations in hematologic malignancies.//Am J Phys Med Rehabil. 2011. Vol. 90, N 5 Suppl 1. P. S88 - 94.
75. Reda G. et al. Secondary Malignancies in Chronic Lymphocytic Leukemia: A Single Centre Retrospective Analysis of 514 Cases//Blood. 2015. Vol. 126, N 23.
76. Beiggi S. et al. Increased risk of second malignancies in chronic lymphocytic leukaemia patients as compared with follicular lymphoma patients: a Canadian population-based study.//Br J Cancer. 2013. Vol. 109, N 5. P. 1287 - 1290.
77. Wo owiec D. et al. Safety and Efficacy of Bendamustine Monotherapy in the First-Line Treatment of Patients with Chronic Lymphocytic Leukemia: Polish Lymphoma Research Group Real-Life Analysis//Chemotherapy. 2019. Vol. 64, N 3. P. 155 - 162.
78. Baltasar P. et al. Efficacy and Safety of Treatment Venetoclax Monotherapy or Combined with Rituximab in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL) in the Real-World Setting in Spain: The Venares Study//Blood. 2021. Vol. 138, N Supplement 1. P. 1561 - 1561.
79. Oken M.M. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group//Am J Clin Oncol. 1982. Vol. 5, N 6. P. 649 - 655.
80. Linn B.S., Linn M.W., Gurel L. Cumulative illness rating scale//J Am Geriatr Soc. 1968. Vol. 16, N 5. P. 622 - 626.
81. D. Catovsky, S. Richards, E. Matutes, et al., UK National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group; NCRI Chronic Lymphocytic Leukaemia Working Group Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 trial): a randomised controlled trial Lancet, 370 (2007), pp. 230 - 239.
- Гражданский кодекс (ГК РФ)
- Жилищный кодекс (ЖК РФ)
- Налоговый кодекс (НК РФ)
- Трудовой кодекс (ТК РФ)
- Уголовный кодекс (УК РФ)
- Бюджетный кодекс (БК РФ)
- Арбитражный процессуальный кодекс
- Конституция РФ
- Земельный кодекс (ЗК РФ)
- Лесной кодекс (ЛК РФ)
- Семейный кодекс (СК РФ)
- Уголовно-исполнительный кодекс
- Уголовно-процессуальный кодекс
- Производственный календарь на 2025 год
- МРОТ 2026
- ФЗ «О банкротстве»
- О защите прав потребителей (ЗОЗПП)
- Об исполнительном производстве
- О персональных данных
- О налогах на имущество физических лиц
- О средствах массовой информации
- Производственный календарь на 2026 год
- Федеральный закон "О полиции" N 3-ФЗ
- Расходы организации ПБУ 10/99
- Минимальный размер оплаты труда (МРОТ)
- Календарь бухгалтера на 2025 год
- Частичная мобилизация: обзор новостей
- Постановление Правительства РФ N 1875